Abstract
Focal epithelial hyperplasia (FEH) is a human papillomavirus (HPV)-induced alteration of the oral mucosa that presents with a clinically distinct appearance. While other HPV-infected lesions such as squamous papilloma, verruca vulgaris, and condyloma acuminatum involve the skin, oral mucosa, and genital mucosa, FEH occurs only in the oral mucosa. The affected oral mucosa exhibits multiple papules and nodules with each papule/nodule being flat-topped or sessile. The affected region resembles the normal color of oral mucosa rather than appearing as a white color since the epithelial surface is not hyperkeratinized. Almost all cases present with multiple sites of occurrence. This rare, benign epithelial proliferation is related to low-risk HPV, especially HPV-13 and -32, and is not transformed into carcinoma. We report a case of FEH that arose on the attached gingiva of an East Asian male adult related to prosthesis without detection of any HPV subtype in HPV DNA chip and sequencing.
Focal epithelial hyperplasia (FEH), or Heck's disease, is an uncommon asymptomatic proliferation of the oral mucosa that was first introduced into dermatology literature in 1965 by Archard et al.1 It usually presents in young Native Americans, from Central or South America, as multiple pinkish plaques on the oral mucosa, particularly the lower lip, gingiva, tongue or buccal mucosa. Although the condition was originally described in North American Indians and Eskimos, it has since been reported in a wide variety of other ethnic groups.2
Many reports about FEH were related to human papillomavirus (HPV) infection, frequently to the subtype of 13 and 32. HPV 32 tends to cause the disease in older people, while HPV 13 seems to be equally involved in the both young and old patients.2,3 Treatment is not usually indicated as the lesions may undergo spontaneous regression particularly in children.4
This report is about the rare case of an East Asian male patient complaining of multiple pinkish gingival nodules near the attached gingiva of a recently delivered prosthesis. This unique case was reviewed from the brief history of the patient to DNA chip analysis of HPV subtype. The possible causes of FEH not related to HPV infection or ethnic prevalence may be identified with literature reviews.
A 53-year-old Korean man presented with gingival swelling and multiple small nodules of the right maxilla that involved the buccal attached gingiva from the canine to the second molar. The enlarged gingival surface was pebbly and white or slightly reddish, without hemorrhage or ulceration (Fig. 1A). He also had a small mucosal alteration on the lingual gingiva of the left mandibular first molar, similar to the upper lesion (Fig. 1B). These had first been noticed one month earlier, and the dentist referred him to our dental hospital.
Panoramic radiograph revealed nothing but generalized alveolar bone loss and a periapical radiolucency of the right maxillary lateral incisor (Fig. 1C). He had received prosthetic treatments in the right upper molar area 13 months ago and in the left lower molar area 7 months ago. Since the lesions had developed several months ago, both had slowly enlarged. Routine laboratory parameters with immune parameters, such as B cells, T cells, CD4+, CD8+ and IgE, were normal. The patient was generally healthy and was not a smoker. For a clinical differential diagnosis with arteriovenous malformation (AVM), squamous papilloma and verrucous cancer, an excisional biopsy was performed under local anesthesia. There was no severe bleeding, and no alveolar bone destruction. One week after the removal of the periodontal dressing materials of Coe-Pak®(GC Co., Tokyo, Japan), the denuded alveolar bone healed secondarily.
Under the approval by Institutional Review Board of Seoul National University Dental Hospital, the removed specimen was fixed in 10% neutral formalin, embedded in paraffin, and sectioned into 4 µm thicknesses. Microsections were stained with hematoxylin and eosin, followed by immunohistochemical staining using antibodies targeting Ki-67 and p53 with an indirect triple sandwich method.5 Upon microscopic examination, the excised mucosa was proliferating in a papillary pattern with acanthosis. Rete ridges were widened and elongated but were not psoriasiform (Fig. 1D). Chronic mild inflammation was also observed. There were a few mitosoid cells among the normal keratinocytes in the stratum spinosum (Fig. 1E, Fig. F). No brisk mitotic figures were seen in the stratum basale. Ki-67 was immunoreactive in the suprabasal cell layer as well as the basal cell layer, indicating the hyperplasia of keratinocytes (Fig. 2A). P53-positive cells were scattered within the epithelium but were few in number (Fig. 2B).
For the detection of a HPV subtype infection, 5 - 10 mm3-sized specimen from the central portion of the main lesion were processed on the HPV DNA chip assay (MY-HPV chip kit®, MyGene Co., Seoul, Korea) PCR-based microarray system. This HPV DNA chip contains 20 type-specific probes: 7 low-risk types (6, 11, 34, 40, 42, 43, and 44) and 11 high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 54, and 56) with the additional types of 13 and 32. No HPV DNA was detected on these chip slides (Fig. 2C, Fig. D). The final diagnosis of FEH was established based on the histopathological findings. The patient was followed-up for 18 months with no signs of recurrence.
FEH is a benign epithelial proliferative lesion that involves the oral mucosa. Even though it is typically papulonodular in shape and arises on the labial and buccal mucosa, the papillomatous variant of FEH tends to arise on the masticatory mucosa including attached gingiva and tongue,2 which is consistent with the present case. Based on the clinical manifestation, the differential diagnoses of FEH include various multinodular or verrucous lesions of the oral cavity, such as common wart, verruciform xanthoma, verrucous carcinoma (Ackerman's tumor), multiple endocrine neoplasia syndrome 2B, Cowden syndrome, and Crohn's disease.6,7
Biopsy is still considered to be the gold standard for definitive diagnosis of FEH.2 Although not every FEH case presents a mitosoid cell, it is a pathognomic microscopic feature of FEH.3 The mitosoid cells are virus-altered keratinocytes with nuclei resembling mitotic figures. They are usually present within the spinous cell layer, but can be observed at any levels of the epithelium.8,9 Because this nuclear change is significant for diagnosis, previous studies have recommended a serial section of paraffin block to detect mitosoid bodies,2,3 and we could diagnose the present case based on the presence of this typical finding. In addition, although the mechanism of this unique alteration in the keratinocytes is still unclear, the mitotic figure might reflect a real mitosis of keratinocyte since the mitosoid bodies was positive for Ki-67 in the present study.
The HPV-13 and -32 were first discovered in series of FEH specimens,10,11 and it is currently known that they are responsible for more than 90% of FEHs.6 Therefore, the identification and typing of HPVs can be helpful to differentiate FEH from other HPV-induced lesions. Among widely used techniques, general primer PCR is utilized to identify the presence of HPVs, and additional type-specific PCR, direct DNA sequencing, and type-specific hybridization can be performed to establish subtypes of HPV.6,8,12,13,14 DNA chip assay, which is also based on PCR method, has high sensitivity to identify HPV type and detects single and multiple infections at once.15 However, despite the typical microscopic findings for FEH, we could not find evidences of HPV infection on the HPV DNA chip assay in the present case.
The possible cause of FEH in this patient would include viral infection, habitual irritation with tooth brushing, genetic variations, tobacco chewing, lack of vitamin K, galvanic electricity from dental amalgams, and ill-fitting prosthesis.16 Since the porcelain-fused to metal (PFM) bridge in the right upper molar area and left lower area had been delivered, small and multiple nodules were observed to be enlarged and expanded from margins of the prosthesis to the attached gingiva at a somewhat fast rate.
All the alloys used in dentistry can cause mechanical and electrochemical irritations, which may cause oral lesions. The intensity of the galvanic effect is determined by the difference of the electrode potentials between the casual metals, and is further influenced by the creation and function of passivation layers on the metal-electrolyte interface. An ionic release from dental metallic materials can cause local or general pathological problems by galvanism in sensitive and genetically susceptible individuals.17 The release of metal ions from the dental alloys depends not only on their composition but also significantly on the quality of their processed materials. The speed of this ionic release is a function of the corrosion rate of the alloy and the solubility of the initial corrosion products. In addition, the patient's prosthesis had deep restoration margin (sub-gingival margin) which risks invading soft tissue attachment of the gingiva to the tooth and inducing the galvanic current on the periosteum, often leading to more inflammatory response such as bleeding, swelling and gingival hyperplasia.18 The heavy pontic design of the patient's bridge including the right upper first and second molar also may be irritation to the gingival epithelium. Therefore, it can be presumed that FEH in this East Asian male was caused by the release of metal ions from the recently fabricated PFM bridges with a weak viral infection of type such as HPV 13 or 32.
Treatment of FEH is not always indicated as the lesions are asymptomatic and often regress spontaneously. For the pathological confirmation of the diagnosis, surgical excision involving cryosurgery, electrocoagulatory, and CO2 lasers has been considered the first treatment of choice. The long-term treatment results of FEH are typically acceptable, and there is no evidence that FEH has malignant potential or that immunodeficiency would predispose patients susceptible to FEH.2,3
In conclusion, FEH is a benign disease having no malignant potential. One case of FEH that arose on the attached gingiva of an East Asian male adult related to his prostheses was reviewed. A metal ionic release from the recently fabricated PFM bridges was considered to be the main cause of FEH in the sensitive and genetically susceptible patient. Although there was no detection of the HPV virus in DNA chip assays, these results will have important implications in the explanation of the etiology of FEH in the oral cavity.
Figures and Tables
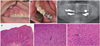 | Fig. 1Clinical intraoral view of the elevated nodules in the right upper posterior buccal gingiva (A) and in the left lower lingual attached gingiva (B) with a panoramic radiograph (C). A papillary proliferating pattern with acanthosis. Rete ridges were widened and elongated, but were not psoriasiform (original magnification, ×12.5) (D). Stratum spinosum layer showing a few mitosoid cells among the normal keratinocyte without brisk mitotic figures (original magnification, ×400) (E, F). |
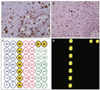 | Fig. 2Immunohistochemical results of Ki-67 and p53. Ki-67 was immunoreactive in the cells of the suprabasal layer as well as the basal cell layer, indicating hyperplasia of the keratinocytes (A), and p53-positive cells were scattered within the epithelium, but were few in number (B) (original magnification, ×400). Mitosoid cells were positive for Ki-67, but negative for p53. All the negative results of the DNA chip analysis (C, D). |
References
1. Archard HO, Heck JW, Stanley HR. Focal epithelial hyperplasia: an unusual oral mucosal lesion found in indian children. Oral Surg Oral Med Oral Pathol. 1965; 20:201–212.
2. Said AK, Leao JC, Fedele S, Porter SR. Focal epithelial hyperplasia - an update. J Oral Pathol Med. 2013; 42:435–442.
3. Jayasooriya PR, Abeyratne S, Ranasinghe AW, Tilakaratne WM. Focal epithelial hyperplasia (Heck's disease): report of two cases with PCR detection of human papillomavirus DNA. Oral Dis. 2004; 10:240–243.
4. Maschke J, Brauns TC, Goos M. Imiquimod for the topical treatment of focal epithelial hyperplasia (Heck disease) in a child. J Dtsch Dermatol Ges. 2004; 2:848–850.
5. Kim SM, Myoung H, Choung PH, Kim MJ, Lee SK, Lee JH. Metastatic leiomyosarcoma in the oral cavity: case report with protein expression profiles. J Craniomaxillofac Surg. 2009; 37:454–460.
6. Bennett LK, Hinshaw M. Heck's disease: diagnosis and susceptibility. Pediatr Dermatol. 2009; 26:87–89.
7. AlSheddi MA, Faden AA. Multifocal epithelial hyperplasia in an adult female. Hong Kong J Dermatol Venereol. 2012; 20:23–27.
8. Padayachee A, van Wyk CW. Human papillomavirus (HPV) DNA in focal epithelial hyperplasia by in situ hybridization. J Oral Pathol Med. 1991; 20:210–214.
9. Ledesma-Montes C, Vega-Memije E, Garcés-Ortíz M, Cardiel-Nieves M, Juárez-Luna C. Multifocal epithelial hyperplasia. Report of nine cases. Med Oral Patol Oral Cir Bucal. 2005; 10:394–401.
10. Pfister H, Hettich I, Runne U, Gissmann L, Chilf GN. Characterization of human papillomavirus type 13 from focal epithelial hyperplasia Heck lesions. J Virol. 1983; 47:363–366.
11. Beaudenon S, Praetorius F, Kremsdorf D, Lutzner M, Worsaae N, Pehau-Arnaudet G, Orth G. A new type of human papillomavirus associated with oral focal epithelial hyperplasia. J Invest Dermatol. 1987; 88:130–135.
12. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003; 16:1–17.
13. Falaki F, Amir Chaghmaghi M, Pakfetrat A, Delavarian Z, Mozaffari PM, Pazooki N. Detection of human papilloma virus DNA in seven cases of focal epithelial hyperplasia in Iran. J Oral Pathol Med. 2009; 38:773–776.
14. Ozden B, Gunduz K, Gunhan O, Ozden FO. A Case Report of Focal Epithelial Hyperplasia (Heck's disease) with PCR Detection of Human Papillomavirus. J Maxillofac Oral Surg. 2011; 10:357–360.
15. Choi YD, Jung WW, Nam JH, Choi HS, Park CS. Detection of HPV genotypes in cervical lesions by the HPV DNA Chip and sequencing. Gynecol Oncol. 2005; 98:369–375.
16. Ledesma-Montes C, Garces-ortiz M, Edmundo B. Multifocal epithelial hyperplasia. An unusual lesion. Webmedcentral Pathology. 2012; 3:WMC003003.
17. Ciszewski A, Baraniak M, Urbanek-Brychczyńska M. Corrosion by galvanic coupling between amalgam and different chromium-based alloys. Dent Mater. 2007; 23:1256–1261.
18. Padbury A Jr, Eber R, Wang HL. Interactions between the gingiva and the margin of restorations. J Clin Periodontol. 2003; 30:379–385.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download