Abstract
PURPOSE
The porcelain fused to gold has been widely used as a restoration both with the natural esthetics of the porcelain and durability and marginal fit of metal casting. However, recently, due to the continuous rise in the price of gold, an interest towards materials to replace gold alloy is getting higher. This study compared the bond strength of porcelain to millingable palladium-silver (Pd-Ag) alloy, with that of 3 conventionally used metal-ceramic alloys.
MATERIALS AND METHODS
Four types of metal-ceramic alloys, castable nonprecious nickel-chrome alloy, castable precious metal alloys containing 83% and 32% of gold, and millingable Pd-Ag alloy were used to make metal specimens (n=40). And porcelain was applied on the center area of metal specimen. Three-point bending test was performed with universal testing machine. The bond strength data were analyzed with a one-way ANOVA and post hoc Scheffe's tests (α=.05).
RESULTS
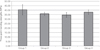
The 3-point bending test showed the strongest (40.42 ± 5.72 MPa) metal-ceramic bond in the nonprecious Ni-Cr alloy, followed by millingable Pd-Ag alloy (37.71 ± 2.46 MPa), precious metal alloy containing 83% of gold (35.89 ± 1.93 MPa), and precious metal alloy containing 32% of gold (34.59 ± 2.63 MPa). Nonprecious Ni-Cr alloy and precious metal alloy containing 32% of gold showed significant difference (P<.05).
Metal-ceramic restoration is an excellent restoration which combines the natural esthetics of veneering porcelain with the durability and the marginal fit of metal casting, and it has been widely used in clinic since it was developed in the early 1950s.1,2,3 Precious and non-precious metal alloys are used in this restoration. Among precious metal alloys, gold alloy is mostly used for its good castability, easiness of polishing, high ductility, malleability and corrosion resistance.4 However, continuous rise in gold price in the past 40 years led to higher manufacturing cost. On the other hand, among non-precious metal alloys, nickel-chrome (Ni-Cr) alloy is mostly used for its excellent mechanical properties.5,6 However, because of its flaws such as poor biocompatibility and corrosion resistance, clinical application of Ni-Cr alloy to dental restorations is controvertible.7,8 In metal-ceramic restorations, application of opaque porcelain is required to block the color of metal coping, which leads to limitations of natural color expression,9 and exposure of metal colors in cervical area causes esthetic problems.10,11
To compensate these limitations of metal-ceramic restorations, and as the demand for esthetics of restorations increases, clinical application of all-ceramic restoration has increased. In all-ceramic restorations, use of materials with similar colors to those of tooth made it possible to fabricate highly esthetic restorations. However, problems such as lower marginal fit12 and frequent fracture in connector area because of low strength have been reported.13,14
Meanwhile, interest in development of CAD/CAM (Computer Aided Design/Computer Aided Manufacturing) system and application of the system to dentistry for fabrication of prosthesis has been increased lately. Past metal-ceramic restorations made by casting method had possibilities of damage and deformation during casting process. Especially, when the span of restoration gets longer, the possibility of causing precision problems in prosthesis increases.15 On the other hand, CAD/CAM system makes it possible to fabricate restorations with high precision regardless of span of the restoration.16 Materials used in fabrication of prosthesis with CAD/CAM system include titatnium, zirconia, etc. Titanium is highly biocompatible, but has problems of low bond strength to porcelain.17 Zirconia, as other all-ceramic restorations, is highly esthetic and also has high strength and precision, but still has problems such as veneer porcelain chipping.18,19
Therefore, palladium-silver (Pd-Ag) alloy which is highly biocompatible and millingable has been developed recently. Since 1970s, Pd-Ag alloy has been used and numerous researches concerned with palladium reported that alloy with higher palladium content showed similar response to 22K gold in vivo.20,21,22 Recently, developed Pd-Ag alloy (Innovium®; Ceragem Biosys, Ilsan, Korea) shows sufficient yield strength (390 MPa) and low density (10.93 g/cm3) because of high portion of palladium. However, it has higher coefficient of thermal expansion (CTE; 16.6±0.5 10-6/℃) than conventional metal-ceramic alloys, and this can affect the bonding between the alloy and porcelain used in conventional metal-ceramic restoration. But porcelain exclusively adequate to Innovium® has not been developed and studies about bonding strength between Innovium® and conventional porcelain are insufficient.
In this study, bond strength of newly developed millingable Pd-Ag alloy to porcelain was analyzed and compared with those of nonprecious Ni-Cr alloy, precious metal alloy with high gold content, and alternative precious metal alloy with low gold content by 3-point bending test. The null hyposthesis was that millingable Pd-Ag alloy has similar bond strength to porcelain with other metal-ceramic alloy.
Four types of metal-ceramic alloys were used. For castable nonprecious Ni-Cr alloy, VeraBond® 2V (Aalbadental Inc., Fairfield, CA, USA) was used, and for castable precious metal alloy, V-Supragold® (Cendres+Me'taux SA, Biel/Bienne, Switzerland) containing 83% of gold and Esteticor Implant® 32 (Cendres+Me'taux SA, Biel/Bienne, Switzerland) containing 32% of gold were used. Innovium® was used as millingable Pd-Ag alloy, and ZEO CE Light® (Yamamoto Precious Metal Co., Osaka, Japan) was used as porcelain powder. Each metal-ceramic alloy was classified into Group 1 to 4 in order (Table 1).
Thirty plastic patterns with size of 26.0 × 4.0 × 0.6 mm were made to fabricate castable metal-ceramic alloy specimens. Sprues were made for plastic patterns. Plastic patterns were invested with Phosphate-bonded investment (UNI-VEST; Shofu Inc., Kyoto, Japan) which contains silica as main fire retardant. After burn out of the invested patterns, VeraBond® 2V, V-Supragold®, Esteticor Implant® 32 were cast for each 10 patterns following manufacturer's instruction. After removal of sprues from casted metal specimens, casted specimens were trimmed with carbide bur and SiC paper into 25.0 × 3.0 × 0.5 mm sized specimens to meet ISO 9693 standards.23 The thickness of the specimens was verified using a digimatic micrometer MDC-25PJ (Mitutoyo CO., Kawasaki, Japan). To fabricate millingable Pd-Ag alloy specimens, Innovium® alloy were milled into 25.0 × 3.0 × 0.5 mm size.
The airborne-particle abrasion was performed for 20 seconds using 110 µm alumina oxide (Al2O3) particles at 3 bars air pressure at a distance of 20 mm from metal speciemen to increase the surface area and enhance porcelain wetting. After this procedure, ultrasonic cleaning and steam cleaning were carried out (Fig. 1).
Porcelain layer with size of 8.0 × 3.0 × 1.1 mm was placed in the center area of manufactured metal specimens according to the ISO 9693 standards.23 In Group 1, 2, 3, firing was performed according to the recommendation from the porcelain manufacturer. In Group 4, the firing was accomplished with regard to the recommendation from the manufacturer of alloy (Table 2). Degassing procedure was performed for Group 1, 2, 3 specimens and oxidation procedure instead of degassing was performed for Group 4 specimens, following manufacturer's order. The entire build-up process followed the porcelain manufacturer's instructions and beginning with the application of 2 uniform coats of opaque porcelain using a brush on the porcelain-bearing area. The dentin body porcelain was formed on the opaque layer using a metal matrix to achieve uniform thickness of porcelain and distinct a porcelain-metal margin. The firing shrinkage was compensated by applying a second layer of body porcelain, making a total thickness of porcelain to 1.1 mm. Finally, a glaze layer was applied and fired. Porcelain application for 40 specimens was performed by a single technician.
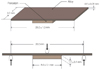
The bond strength between metal-ceramic alloys and porcelain was measured by a 3-point bending test on a universal testing machine Instron 5583 (Instron Co., Norwood, MA, USA). The specimen was positioned on a specially fabricated metal supports (20 mm of distance) with the porcelain facing downward. The metal supports were used to align and stabilize the specimen. A compressive load was applied at the midpoint of the metal specimen with a rounded-tip loading rod of 1 mm radius at a crosshead speed of 1.5 mm/min until a sudden drop in load occurred in the load-deflection curve, indicating the bond failure (Fig. 2).
The failure load was recorded digitally with a personal computer using software provided by the testing machine manufacturer. The bond strength was calculated by the following equation given in ISO 9693 standards.23
F represents measured load, k is a constant can be calculated numerically on the basis of the flow chart in ISO 9693 standards23 with units of mm-2. The value of k depends on the modulus of elasticity and the thickness of specimen, and Σ represents bond strength.
The SPSS (Release 18.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were analyzed by one-way analysis of variance (ANOVA). When significant difference was detected among the means, post hoc Scheffe's test was applied. All tests were conducted at the 95% level of confidence (α=.05).
The bond strength of porcelain to millingable Pd-Ag alloy showed no significant difference from that of other metal-ceramic alloy. Therefore, the null hypothesis that millingable Pd-Ag alloy has similar bond strength to porcelain to other metal-ceramic alloy was accepted.
The use of alternative metal-ceramic alloys is on the rise to replace gold alloy for economic reasons. A nobel Pd-Ag alloy which has millingable characteristic offers high biocompatibility and physical properties, and because this alloy is made into shape by CAD/CAM system, there are less chances of damage or deformity which may be caused by casting. However, researches about clinical application of this alloy in dental restoration are yet insufficient. This study was undertaken to evaluate the bond strength of porcelain to millingable Pd-Ag alloy, as compared to conventionally used metal-ceramic alloys.
Due to the low tensile, shear strength of porcelain and the vulnerability to impacts, one of the fundamental requirements of clinically successful metal-ceramic restorations is to obtain strong bonding between the metal coping and the veneering porcelain. When the veneering porcelain is fractured due to the failure of metal-ceramic bonding, time and cost to re-make the restoration are needed, which has clinical significance. The mechanisms involved in metal-ceramic bonding are as follows: chemical bonding, mechanical bonding, van der Waal's force, and the bonding from compressive force generated by the CTE difference between the metal and the porcelain.24,25 Porcelain fracture is caused by several factors: discrepancy of CTE between metals and porcelain, microcracks occurring from condensation and sintering process of porcelain, and occlusal forces or trauma.26
The CTE of commercially used porcelains ranges 13.0-14.0 × 10-6/℃. Microcrack and bond strength loss of porcelain can occur when the CTE difference between metal and porcelain is over 1.7-2.2 × 10-6/℃. To prevent the fracture, the CTE of porcelain needs to be lower than that of metal as much as 0.5-1.0 × 10-6/℃. Bonding at the metal-ceramic interface becomes weaker when the two CTE values are not approximate, which can be concluded that especially under the temperature of 600℃ where porcelain easily cracks, the CTE of metal should be close to that of porcelain.27 The CTE of metal-ceramic alloys should range 13.7-15.0 × 10-6/℃ to correspond with the recommended range of the manufacturer for the porcelain used in this study. The CTE of Group 1 (13.7 × 10-6/℃), Group 2 (14.2 × 10-6/℃), Group 3 (14.2 × 10-6/℃) all corresponded to the recommendation. In contrast, the Group 4 (16.6 ± 0.5 10-6/℃) had higher CTE than other metal-ceramic alloys and was out of the recommended range by the manufacturer. No porcelains in the current market has the required CTE values to be used with millingable Pd-Ag alloy.
Shell and Nielsen28 reported that the chemical bonding was the most important among the bonding mechanisms of metal and porcelain, whereas the mechanical bonding had no significance. It is known that chemical bonding is influenced by the formation of an oxide layer on the metal-ceramic interface, and this is affected by the composition of alloy. The oxide layer has been studied extensively and shown to play an important role in chemical bonding. Chemical bonding results from covalent or ionic bond between the oxides which are diffused from the metal surface and the oxides inside the porcelain. It is also recognized that metal-ceramic interface with proper oxide layer has higher resistance to bonding failure, whereas thin oxide layer can be completely removed during the firing of porcelain, and excessively thick oxide layer can produce weaker bond strength due to the low cohesive strength of oxide layer.29,30,31 Because gold produces unstable oxide layers incorporating some elements into gold alloys such as tin and indium can help forming stable oxide layers, hence increasing the bond strength of porcelain.32 Lee et al.33 reported that it was favorable to perform degassing in order to obtain proper oxide layer, so degassing was performed in nonprecious Ni-Cr alloy and precious metal alloys containing 83% and 32% of gold Group. Millingable Pd-Ag alloy tends to produce excessively thick oxide layer at firing temperatures above 950℃, which required a different oxidation process recommended by the manufacturer instead of the standard degassing procedure. Therefore, porcelain which required a final firing temperature lower than 950℃ and was able to show clinically successful results when applied to other alloys was used in this experiment.
Also, increasing the surface roughness of the alloys can improve the bond strength. Airborne-particle abrasion increases the surface energy of the alloys and the wettability of the porcelain.34 Most commonly used air-abrasion particles for this purpose are Al2O3 particles. Külünk et al.35 used several air-abrasion particles to determine the effects of the particle size and types on the metal-ceramic bond strength, and reported that 110 µm Al2O3 particles showed the highest bond strength. Therefore, the metal specimen in this study were all sandblasted with 110 µm Al2O3 particles with 3-bar air pressure to increase surface roughness.
Because of the complexity of metal-ceramic bonding characteristics, metal-ceramic bond strength could be tested with various methods such as shear bond test and three- or four-point bending tests. Anusavice et al.36 and Lenz et al.37 concluded that there is no ideal testing method, since the different morphology of specimens from different tests can induce various pattern of stress concentration, which may lead to different bond strength. As a result of similar study, Papazoglou and Brantley38 also reported that there was no absolute consistency among the results from different testing methods. According to a critical analysis by Hammad and Talic39 on the bond strength test of metal-ceramic system, shear bond strength test with a flat interface can only measure the forces directly imposed on the interface, making it impossible to evaluate the modulus of elasticity of metal which is taken into account in bending test. Therefore, in the current study, three-point bending test was performed in accordance with the ISO 9693 standards,23 which has repeatability, quantification, easy specimen making and testing procedure, and the thickness of porcelain layer and metal plate that is clinically acceptable.40 The mean debonding/crack initiation strength should be above 25 MPa to meet ISO standards.23
To confirm whether the values from the experiment show a normal distribution, K-S test (Kolmogorov-Smirnov test) was performed, and to compare the bonding strength among the types of metal-ceramic alloys, one-way ANOVA test was used, which demonstrated statistically significant difference among the 4 groups (P<.05). To verify the significance between the groups, post hoc Scheffe's test was performed. Only between the Group 1 and Group 3 showed statistically significant difference (P<.05), while there was no statistically significant difference among other groups.
In this study, the bond strength between metal-ceramic alloys and porcelain ranges from 34.59 to 40.42 MPa, non-precious Ni-Cr alloy Group showed the strongest (40.42 ±5.72 MPa) metal-ceramic bond, followed by millingable Pd-Ag alloy (37.71 ± 2.46 MPa), precious metal alloy containing 83% of gold (35.89 ± 1.93 MPa), and precious metal alloy containing 32% of gold (34.59 ± 2.63 MPa). This result is similar to other researches reported earlier. Barghi et al.32 reported that Ni-Cr alloys had higher bond strength to porcelain than gold alloys.
In Group 1, 2, 3 of this study, debonding of porcelain showed a pattern of a propagation starting from one end of a specimen. This corresponds with the previous results where compressive force is the highest at the terminal area of metal-ceramic interface, as reported by Anusavice et al.36 and Lenz et al.37 However, in Group 4, the debonding of porcelains occurred simultaneously over the entire specimens, which was considered to be affected by the CTE of the alloy which was out of the recommended range.
In order to secure proper bond strength between the metal-ceramic alloys and porcelain, it is crucial to design metal coping so as to provide adequate thickness of porcelain and sufficient support.41 Also, removing surface reaction layer (α-case layer) of the metal cast and proper metal surface treatments (e.g, airborne-particle abrasion) and heat treatment of the metal surface to form proper oxide layer are needed before the porcelain build-up.32,42,43 When needed, the usage of bonding agent prior to the application of opaque porcelain can inhibit the formation of excessive oxide layer on the metal surface during porcelain firing process, hence improving the bond strength.44 It is also necessary to conduct the firing schedule with respect to the manufacturer's recommendation using an exclusive porcelain which has similar CTE.27,45,46
From the results above, the type of metal-ceramic alloys significantly affected the bond strength, while all metal-ceramic alloys showed clinically applicable bond strength. It is considered that comparative studies are required concerning the bond strength between metal-ceramic alloy and porcelain upon the types of veneering porcelains, surface treatments of metals, existence of thermocycling, and the firing temperature. In case of millingable Pd-Ag alloy, it is considered that developing porcelain with approximate CTE is required for higher bond strength.
Within the limitations of this investigation and for the materials used in this study of the bond strength of porcelain to millingable Pd-Ag alloy, the results support the following conclusions:
The type of metal-ceramic alloys affects the bond strength of porcelain. Non-precious Ni-Cr alloy showed significantly increased bond strength to porcelain compared with precious metal alloy containing 32% of gold. However, the bond strength of porcelain to millingable Pd-Ag alloy showed no significant difference compared to other metal-ceramic alloy. Every metal-ceramic alloy used in this study showed clinically applicable bond strength with porcelain.
Figures and Tables
Table 1
Metal ceramic alloys used in this study

Table 2
Firing schedule of porcelain

Table 3
Mean and standard deviation for bond strength (Unit : MPa)

| Group | Mean ± SD | N |
|---|---|---|
| 1 | 40.42 ± 5.72 | 10 |
| 2 | 35.89 ± 1.93 | 10 |
| 3 | 34.59 ± 2.63 | 10 |
| 4 | 37.71 ± 2.46 | 10 |
References
1. Brecker SC. Porcelain baked to gold-A new medium in prosthodontics. J Prosthet Dent. 1956; 6:801–810.
2. Bagby M, Marshall SJ, Marshall GW Jr. Metal ceramic compatibility: a review of the literature. J Prosthet Dent. 1990; 63:21–25.
3. Kim CM, Lee JH, Cho IH. A study on the bond strength of non-precious alloys used for the porcelain fused to metal crown. J Korean Acad Stomatognathic Funct Occlusion. 2006; 22:203–210.
4. Kim I, Yang HS. A study on the bond strength between reused dental alloys and porcelain. J Korean Acad Prosthodont. 1993; 31:181–190.
5. do Prado RA, Panzeri H, Fernandes Neto AJ, das Neves FD, da Silva MR, Mendonça G. Shear bond strength of dental porcelains to nickel-chromium alloys. Braz Dent J. 2005; 16:202–206.
6. Moffa JP, Lugassy AA, Guckes AD, Gettleman L. An evaluation of nonprecious alloys for use with porcelain veneers. Part I. Physical properties. J Prosthet Dent. 1973; 30:424–431.
7. Kelly JR, Rose TC. Nonprecious alloys for use in fixed prosthodontics: a literature review. J Prosthet Dent. 1983; 49:363–370.
8. Morris HF. Veterans Administration Cooperative Studies Project No. 147. Part IV: Biocompatibility of base metal alloys. J Prosthet Dent. 1987; 58:1–5.
9. Spear F, Holloway J. Which all-ceramic system is optimal for anterior esthetics? J Am Dent Assoc. 2008; 139:19S–24S.
10. Magne P, Magne M, Belser U. The esthetic width in fixed prosthodontics. J Prosthodont. 1999; 8:106–118.
11. O'Boyle KH, Norling BK, Cagna DR, Phoenix RD. An investigation of new metal framework design for metal ceramic restorations. J Prosthet Dent. 1997; 78:295–301.
12. Stappert CF, Dai M, Chitmongkolsuk S, Gerds T, Strub JR. Marginal adaptation of three-unit fixed partial dentures constructed from pressed ceramic systems. Br Dent J. 2004; 196:766–770.
13. Campbell SD, Sozio RB. Evaluation of the fit and strength of an all-ceramic fixed partial denture. J Prosthet Dent. 1988; 59:301–306.
14. Kelly JR, Tesk JA, Sorensen JA. Failure of all-ceramic fixed partial dentures in vitro and in vivo: analysis and modeling. J Dent Res. 1995; 74:1253–1258.
15. Zervas PJ, Papazoglou E, Beck FM, Carr AB. Distortion of three-unit implant frameworks during casting, soldering, and simulated porcelain firings. J Prosthodont. 1999; 8:171–179.
16. Katsoulis J, Mericske-Stern R, Rotkina L, Zbären C, Enkling N, Blatz MB. Precision of fit of implant-supported screw-retained 10-unit computer-aided-designed and computer-aidedmanufactured frameworks made from zirconium dioxide and titanium: an in vitro study. Clin Oral Implants Res. 2014; 25:165–174.
17. Gilbert JL, Covey DA, Lautenschlager EP. Bond characteristics of porcelain fused to milled titanium. Dent Mater. 1994; 10:134–140.
18. Reich S, Wichmann M, Nkenke E, Proeschel P. Clinical fit of all-ceramic three-unit fixed partial dentures, generated with three different CAD/CAM systems. Eur J Oral Sci. 2005; 113:174–179.
19. Bachhav VC, Aras MA. Zirconia-based fixed partial dentures: a clinical review. Quintessence Int. 2011; 42:173–182.
20. Huget EF, Civjan S. Status report on palladium-silver-based crown and bridge alloys. J Am Dent Assoc. 1974; 89:383–385.
21. Goodacre CJ. Palladium-silver alloys: a review of the literature. J Prosthet Dent. 1989; 62:34–37.
22. Kansu G, Aydin AK. Evaluation of the biocompatibility of various dental alloys: Part I-Toxic potentials. Eur J Prosthodont Restor Dent. 1996; 4:129–136.
23. International Organization for Standardization. ISO 9693: metal-ceramic dental restorative systems. 2012.
24. Knap FJ, Ryge G. Study of bond strength of dental porcelain fused to metal. J Dent Res. 1966; 45:1047–1051.
25. Silver M, Klein G, Howard MC. An evaluation and comparison of porcelain-fused-to-cast metals. J Prosthet Dent. 1960; 10:1055–1064.
26. Ozcan M. Fracture reasons in ceramic-fused-to-metal restorations. J Oral Rehabil. 2003; 30:265–269.
27. Fairhurst CW, Anusavice KJ, Ringle RD, Twiggs SW. Porcelain-metal thermal compatibility. J Dent Res. 1981; 60:815–819.
28. Shell JS, Nielsen JP. Study of the bond between gold alloys and porcelain. J Dent Res. 1962; 41:1424–1437.
29. de Melo RM, Travassos AC, Neisser MP. Shear bond strengths of a ceramic system to alternative metal alloys. J Prosthet Dent. 2005; 93:64–69.
30. Mackert JR Jr, Ringle RD, Parry EE, Evans AL, Fairhurst CW. The relationship between oxide adherence and porcelain-metal bonding. J Dent Res. 1988; 67:474–478.
31. McLean JW. The metal-ceramic restoration. Dent Clin North Am. 1983; 27:747–761.
32. Barghi N, McKeehan-Whitmer M, Aranda R. Comparison of fracture strength of porcelain-veneered-to-high noble and base metal alloys. J Prosthet Dent. 1987; 57:23–26.
33. Lee EH, Jeon YC, Jeong CM, Lim JS. Effect of degassing condition on ceramic bond strength of Ni-Cr alloys. J Korean Acad Prosthodont. 2000; 38:461–471.
34. Jochen DG, Caputo AA, Matyas J. Effect of metal surface treatment on ceramic bond strength. J Prosthet Dent. 1986; 55:186–188.
35. Külünk T, Kurt M, Ural Ç, Külünk Ş, Baba S. Effect of different air-abrasion particles on metal-ceramic bond strength. J Dent Sci. 2011; 6:140–146.
36. Anusavice KJ, Dehoff PH, Fairhurst CW. Comparative evaluation of ceramic-metal bond tests using finite element stress analysis. J Dent Res. 1980; 59:608–613.
37. Lenz J, Schwarz S, Schwickerath H, Sperner F, Schäfer A. Bond strength of metal-ceramic systems in three-point flexure bond test. J Appl Biomater. 1995; 6:55–64.
38. Papazoglou E, Brantley WA. Porcelain adherence vs force to failure for palladium-gallium alloys: a critique of metal-ceramic bond testing. Dent Mater. 1998; 14:112–119.
39. Hammad IA, Talic YF. Designs of bond strength tests for metal-ceramic complexes: review of the literature. J Prosthet Dent. 1996; 75:602–608.
40. Barghi N, Lorenzana RE. Optimum thickness of opaque and body porcelain. J Prosthet Dent. 1982; 48:429–431.
41. Warpeha WS Jr, Goodkind RJ. Design and technique variables affecting fracture resistance of metal-ceramic restorations. J Prosthet Dent. 1976; 35:291–298.
42. Lahori M, Nagrath R, Sisodia S, Dagar P. The effect of surface treatments on the bond strength of a nonprecious alloy-ceramic interface: an invitro study. J Indian Prosthodont Soc. 2014; 14:151–155.
43. Li BH, Ye JT, Liao JK, Zhuang PL, Zhang YP, Li JY. Effect of pretreatments on the metal-ceramic bonding strength of a Pd-Ag alloy. J Dent. 2014; 42:319–328.
44. Gilbert JL, Covey DA, Lautenschlager EP. Bond characteristics of porcelain fused to milled titanium. Dent Mater. 1994; 10:134–140.
45. Anusavice KJ, Dehoff PH, Gray A, Lee RB. Delayed crack development in porcelain due to incompatibility stress. J Dent Res. 1988; 67:1086–1091.
46. Rosenstiel SF, Land MF, Fujimoto J. Contemporary fixed prosthodontics. 4th ed. St. Louis, Mo: Mosby Elsevier;2006. p. 743–750.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download