Abstract
PURPOSE
This study evaluated the adhesion to acrylic resin specimens and biofilm formation capability of Candida albicans strains isolated from HIV positive subjects' oral rinse solutions.
MATERIALS AND METHODS
The material tested was a heat-cured acrylic resin (Acron Duo). Using the adhesion and crystal violet assays, 14 oral Candida albicans isolated from HIV-positive subjects and 2 references Candida strains (C. albicans ATCC 90028 and C. albicans ATCC 90128) were compared for their biofilm production and adhesion properties to acrylic surfaces in vitro.
Acrylic resin, in particular poly-(methyl-methacrylate), is a base material for dentures. It is widely used due to its good working properties such as simple preparation and fixing, accuracy of fit, stability in the oral environment, aesthetic aspect and low price.1-4 However, acrylic resin is a polaric molecule.5,6 PMMA absorbs water and polymeric break down occurs, which causes stained and/or malodorous base material.7 Another problem associated with water absorption is the ability of certain organisms to colonize the intaglio surface of an acrylic denture. Loss or gain of water in the surface layers may occur quite rapidly, which contributes to surface crazing.8 Also, irregularities in the acrylic resin surface are a factor in microorganism entrapment.9,10
The acrylic denture, which is ill-fitting and unhygienic, can act as a reservoir of infection.11,12 The main cause of Candida-associated denture stomatitis might be related with Candida species adhesion to oral epithelia and denture acrylic surfaces.11,13-15 Denture stomatitis is one of the most common problem of old denture users, with 25-65% of prevalence rates.15-18
Previous studies showed that one of the risk factor for denture stomatitis is in vivo formation of Candida biofilms.14 These biofilms made up of yeast cells and their hyphae, firmly fixed to the biomaterials and they seem to play a role in the formation of biofilms.14,19 The adjacency of the biofilm and extra polymeric matrix to the oral mucosa induces localized innate inflammatory responses, causing erythema and other clinical symptoms.13
However, denture stomatitis cannot be explained clinically with the presence of biofilm only.13 In fact, fungi such as Candida albicans normally live as innocuous commensal until transformed into parasitic pathogens due to decreased immune response.17,20 It seems that host immune factors also play a role. Although there are some evidence that denture stomatitis is associated with presence of C. albicans, other factors such as denture hygiene, traumatization, bacteria colonization, saliva production deficiency, and certain immune defects may also take a part.14 This is especially important for immune-compromised patients who are infected with human immunodeficiency virus (HIV).12
In HIV-infected individuals, fungi have been increasingly recognized as major pathogens.17 As an increased prevalence of candidal carriage and oral candidiasis is common in cases of HIV infection, oral medical devices, especially dental prosthesis, may be more problematic for these patients because of the tendency for biofilm formation. Some studies have compared the adherence of C. albicans isolates to mucosal surfaces in both HIV-infected patients and HIV-free subjects, as adherence to host surfaces is a prerequisite for subsequent biofilm formation and colonization.21,22 However, there is limited information on the adhesion of C. albicans with associated HIV positive patients' acrylic dentures, this is a hypothesis which is not tested yet. The aim of present study is to determine the properties of biofilm formation and adherence to acrylic dentures of C. albicans strains isolated from HIV positive subjects and compares their properties with standard strains from American Type Culture Collection (ATCC).
In this in vitro study, the tested denture base material was a heat-cured poly methyl-methacrylate (PMMA) acrylic resin (Acron Duo, Associated Dental Products Ltd., Kemdent, Purton, Swindon, Wiltshire, UK). To prepare the PMMA samples, a pink modeling wax (Cavex® Set Up Modeling Wax, Haarlem, The Netherlands) with dimensions of 20 × 20 × 2 mm was placed in hard dental plaster (Moldabaster S, HeraeusKulzer GmBH, Hanau, Germany) in a two-part mold using a standard dental flask. The wax was eliminated under running hot water and the plaster surfaces were sealed with one coat of sealant. The heat-cured acrylic denture base resin was then packed in the molds and the polymerization process was carried out in a water bath at 70℃ for 1 hour followed by boiling for 30 minutes. In order to give as accurate representation as possible of the tissue surface of the dentures, the test sides of the polymerized acrylic resin samples were not polished after demolding. The samples were only washed with water steam under pressure to remove any possible contaminants present on the surfaces and then stored in distilled water at 37℃ for 24 hours, prior to adhesion assays and biofilm formation.
In this in vitro study, 14 C. albicans strains which were chosen from the culture collection of Ankara University Faculty of Medicine, Department of Medical Microbiology Laboratory were evaluated. The strains were isolated from oral rinse samples of HIV positive subjects who were seen at the Infectious Diseases Clinic, Department of Internal Medicine University of Hacettepe between 2002 and 2004. The identity of the isolates was verified again by using a commercially available carbohydrate assimilation tests (API 32C identification system, bioMérieux, France).
C. albicans strains were streaked from their thawed suspension stored at -70℃ onto Sabouraud dextrose agar (SDA, Merck, Germany) and plates are left at 37℃ for 24 hours. A single colony was chosen and inoculated in 25 mL sterile Sabouraud dextrose broth (SDB, Merck, Germany) medium and flasks containing this medium were incubated for 18 hours at 35℃ with agitation (120 rpm). Cells were harvested by centrifugation (6000 rpm, 4℃, 10 minutes) and washed twice with sterile phosphate-buffered saline (PBS, 0.15 mM, pH: 7.2, Ca2+ and Mg2+ free). The yeast cells were resuspended in the same buffer and standardized to a concentration of 1 × 107 and 1 × 106 cfu/mL.
Adhesion assays were performed by using a modification of the technique previously described.23 Briefly, PMMA samples were individually put inside a Petri dish. The standardized C. albicans cell suspension in PBS (1 × 106 cells/mL) was added to each Petri plate covering the acrylic resin material. The cell adhesion was occurred in 2 hours at 37℃. Following incubation, the acrylic resin material gently washed twice with 5 mL PBS thus the non-adherent cells were removed. Afterwards, the adhered yeasts were counted. The method used to do this was to put the PMMA samples in a sterile tube consisting 5 mL saline solution and then mix them using a vortex mixer at the maximum setting for 30s. Following incubation, the acrylic resin material gently washed twice with 5 mL PBS thus the non-adherent cells were removed. Portions (0.1 mL) of dilutions were spread onto SDA and the medium were left for 24 hours at 37℃.
After the incubation period, the yeast colonies were observed and the number of colony forming units (CFU) per milliliter was calculated.
PMMA acrylic specimens without cells were used as negative controls in all experiments. All experiments were performed in triplicate on three separate occasions and evaluated by two independent microbiologists.
Biofilms were showed on pre-sterilized, polystyrene; flat-bottom 96-well microtiter plates (Thermo Scientific, USA). Each well of the microtiter plate was filled with 100 µL of a standardized cell suspension (107 cells/mL). The plate was incubated for 1.5 hours at 37℃ with agitation at 75 rpm thus, the yeast can adhere to the surfaces of the wells.24 For control purposes, three wells of each microtiter plate were prepared under the same conditions except that no Candida suspensions were added. Following the adhesion phase, the cell suspensions were aspirated and the wells were washed twice with 150 µL of PBS solution to remove loosely attached cells. The washed wells were filled with 100 µL SDB medium, and the plates were incubated at 37℃ in a shaker at 75 rpm. The SDB was replaced with fresh medium daily.21 The biofilms were left to develop for up to 48 hours. The biofilm formation was quantified by a modification of a crystal violet assay described by others.25 All assays were done in triplicate on three different occasions.
The biofilm coated wells were washed twice with 200 µL of PBS and were left in room temperature to dry for 45 min. The washed wells were stained with 100 µL of 0.4% crystal violet solution for 45 minutes. Then, each well was washed four times with 350 µL of sterile distilled water to remove crystal violet and quickly destained with 200 µL of 95% ethanol for 45 minutes. After destaining, 100 µL of destaining solution was transferred to a new well and the amount of the crystal violet stain in the destaining solution was measured with a microtiter plate reader (Thermo Labsystems, USA) at 600 nm.21,25
Means and standard deviations were calculated using the Mann-Whitney U test. A P value of less than 0.05 was considered statistically significant.
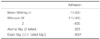
In adhesion assays, similar CFU numbers were calculated by two independent researchers. None of the negative controls exhibited adhesion. In biofilm assays, the HIV-positive subjects' oral isolates and the reference strains were not different from each other and both showed similar absorbance readings. None of the negative controls exhibited biofilm formation. By statistical analysis (Mann-Whitney U test), neither the adhesion assay (P=.52) nor the crystal violet assay (P=.42) revealed significant differences in adhesion and biofilm formation abilities between the two groups of isolates (Table 1, Table 2, Table 3, and Table 4).
Clinically, it is common to find C. albicans biofilms on natural host surfaces or on biomaterials, such as acrylic resins used for the construction of denture bases. Biofilms, consisting of Candida species and bacteria on the denture material, cause inflammation and hyperplasia of the oral mucosa.14,26 Denture stomatitis is the most common form of oral Candida infection among maxillary complete denture wearers with 65% of prevalence rate.27 Oral endogenous bacteria and Candida spp. can easily establish colonies on denture base acrylic resin.28 Acrylic resin denture surfaces colonized by microorganisms might be a reservoir of infection.11,12
C. albicans adhere to polymeric surfaces by Van der Waals and electrostatic forces. The augmentation made by electrostatic and hydrophobic forces for adherence process varies between substrate and environments. In the beginning these forces are important in the initial adhesion and then they cause further bonding and denture plaque formation. Saliva, serum, oral floara and surface topography and chemistry differences may affect this complicated process.29,30
Studies by Morgan and Wilson31 showed that bacteria and fungus affinity to rough acrylic resins. Denture fit surfaces are processed against dental plaster as a representative of oral anatomy. The resultant surface will reproduce some of the surface topography of the stone and therefore will also not be particularly smooth. The intaglio surface of the maxillary denture was more prone to colonization by C. albicans and was not smooth.31 Therefore, in the current study, in order to simulate tissue surface of the dentures, the test sides of the polymerized acrylic resin samples were not polished.
Another predisposing factor for oral mucosal Candida infections is the patient's systemic condition associated with weakened, suppressed, or underdeveloped immune systems.27 Junqueira et al.17 have compared biofilm production of oral and systemic C. albicans isolates from HIV-positive patients, using an in vitro biofilm model on silicone and acrylic resin. Their findings suggest that oral Candida infections are serious infections because the pathogenicity of oral Candida isolates is similar to systemic Candida isolates, so they have the potential to cause systemic infections.
In this study, we compared the properties of oral C. albicans strains isolated from HIV positive subjects and reference strains with respect to their biofilm forming ability and their adherence on acrylic dentures. We used the crystal violet assay for the quantification of biofilm formation. Jin et al.21 compared the XTT reduction assay and the crystal violet assay and their data indicated a significant correlation between the two methods. Since there are several studies indicating that the adhesions of C. albicans to acrylic surfaces may be affected by several factors such as the saliva, serum, oral flora and sucrose rich diet, in vitro nature of the investigation was the limitation of our study.29 Many studies have demonstrated that body fluids, proteins, enzymes, electrolytes and lipids in vivo destroy biomaterials, cause roughness and make it easy for microorganisms to attach to the surfaces.32
It is well known that when we compare with healthy controls, HIV-infected patients may carry C. albicans in their oral environment with a heightened frequency of oral candidiasis without any symptoms.10,26 One possible reason for this may be that the heightened biofilm-forming Candida isolates colonize in the oral cavities of HIV-infected individuals. However, in our study, the statistical analysis shows there is no significant difference in both adhesion and biofilm formation between strains of C. albicans isolated from the oral rinse solutions of HIV infected subjects and reference strains. Our results correlated with the study of Jin et al.21 in that there were no significant quantitative differences in biofilm formation between C. albicans isolates from HIV infected patients and isolates from HIV-free individuals.
According to our results, biofilm formation and adherence abilities of C. albicans to acrylic resin materials do not seem to be related with the cause of denture stomatitis and increased prevalence of candida carriage in HIV-infected individuals. This might be due to the number of control group, which consists of two reference strains and also the limitation of in vitro tests. Further studies with larger groups consisting of healthy and HIV infected subjects are needed to elucidate how in vivo environmental factors interfere with our findings.
Figures and Tables
Table 1
Test statisticsa of adhesion assays
| Adhesion | ||
|---|---|---|
| Mann-Whitney U | 10.000 | |
| Wilcoxon | W | 115.000 |
| Z | −.635 | |
| Asymp Sig. (2 tailed) | .525 | |
| Exact Sig. [ 2 (1 tailed Sig.)] | .600b | |
Table 2
Statistical analysis report of adhesion assays

| Groups | Mean | N | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| 1 | 73.25 | 2 | 78.842 | 73.25 | 18 | 129 |
| 2 | 44.04 | 14 | 33.132 | 39.75 | 1 | 113 |
| Total | 47.69 | 16 | 38.28 | 37.75 | 1 | 129 |
ACKNOWLEDGEMENTS
We thank to Prof. Dr. Alper TEKELİ for providing clinical isolates included in this study and we thank to Iain Maynard for editing English.
References
1. Chen SY, Liang WM. Effects of fillers on fiber reinforced acrylic denture base resins. Mid Taiwan J Med. 2004; 9:203–210.
2. Chen SY, Liang WM, Yen PS. Reinforcement of acrylic denture base resin by incorporation of various fibers. J Biomed Mater Res. 2001; 58:203–208.
3. Jagger DC, Harrison A, Jandt KD. The reinforcement of dentures. J Oral Rehabil. 1999; 26:185–194.
4. Katsikas NG, Huggett R, Harrison A, Vowles RW. The effect of esthetic fibers on the flow properties of an acrylic resin denture base material. Dent Mater. 1994; 10:2–5.
5. Kanie T, Fujii K, Arikawa H, Inoue K. Flexural properties and impact strength of denture base polymer reinforced with woven glass fibers. Dent Mater. 2000; 16:150–158.
6. Miettinen VM, Vallittu PK, Docent DT. Water sorption and solubility of glass fiber-reinforced denture polymethyl methacrylate resin. J Prosthet Dent. 1997; 77:531–534.
7. O'Brien WJ. Dental materials and their selection. 4th ed. Chicago: Quintessence Publishing;2008. p. 9.
8. McCabe JF, Walls A. Applied dental materials. Edinburg: Blackwell Science;1998. p. 105.
9. Aleva NA, Birman EG, Afonso W Jr, Chavasco JK, Paula CR, Ribeiro A, Pereira LJ. Erythematous candidosis in patients with complete dentures and HIV+/AIDS. Mycoses. 2007; 50:407–411.
10. Verran J, Maryan CJ. Retention of Candida albicans on acrylic resin and silicone of different surface topography. J Prosthet Dent. 1997; 77:535–539.
11. Ellepola AN, Samaranayake LP. Adhesion of oral Candida albicans isolates to denture acrylic following limited exposure to antifungal agents. Arch Oral Biol. 1998; 43:999–1007.
12. Perezous LF, Flaitz CM, Goldschmidt ME, Engelmeier RL. Colonization of Candida species in denture wearers with emphasis on HIV infection: a literature review. J Prosthet Dent. 2005; 93:288–293.
13. Ramage G, Coco B, Sherry L, Bagg J, Lappin DF. In vitro Candida albicans biofilm induced proteinase activity and SAP8 expression correlates with in vivo denture stomatitis severity. Mycopathologia. 2012; 174:11–19.
14. Ramage G, Tomsett K, Wickes BL, López-Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 98:53–59.
15. Thein ZM, Samaranayake YH, Samaranayake LP. Characteristics of dual species Candida biofilms on denture acrylic surfaces. Arch Oral Biol. 2007; 52:1200–1208.
16. Chandra J, Kuhn DM, Mukher j, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001; 183:5385–5394.
17. Junqueira JC, Fuchs BB, Muhammed M, Coleman JJ, Suleiman JM, Vilela SF, Costa AC, Rasteiro VM, Jorge AO, Mylonakis E. Oral Candida albicans isolates from HIV-positive individuals have similar in vitro biofilm-forming ability and pathogenicity as invasive Candida isolates. BMC Microbiol. 2011; 11:247.
18. Li L, Finnegan MB, Özkan S, Kim Y, Lillehoj PB, Ho CM, Lux R, Mito R, Loewy Z, Shi W. In vitro study of biofilm formation and effectiveness of antimicrobial treatment on various dental material surfaces. Mol Oral Microbiol. 2010; 25:384–390.
19. Redding S, Bhatt B, Rawls HR, Siegel G, Scott K, Lopez-Ribot J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:669–672.
20. Sweet SP, Cookson S, Challacombe SJ. Candida albicans isolates from HIV-infected and AIDS patients exhibit enhanced adherence to epithelial cells. J Med Microbiol. 1995; 43:452–457.
21. Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol. 2003; 41:2961–2967.
22. Kennedy MJ. Adhesion and association mechanisms of Candida albicans. Curr Top Med Mycol. 1988; 2:73–169.
23. Uludamar A, Ozkan YK, Kadir T, Ceyhan I. In vivo efficacy of alkaline peroxide tablets and mouthwashes on Candida albicans in patients with denture stomatitis. J Appl Oral Sci. 2010; 18:291–296.
24. Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002; 70:878–888.
25. Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol. 2002; 68:2950–2958.
26. Samaranayake LP, Fidel PL, Naglik JR, Sweet SP, Teanpaisan R, Coogan MM, Blignaut E, Wanzala P. Fungal infections associated with HIV infection. Oral Dis. 2002; 8:151–160.
27. Ganguly S, Mitchell AP. Mucosal biofilms of Candida albicans. Curr Opin Microbiol. 2011; 14:380–385.
28. Gornitsky M, ParadisI I, Landaverde G, Malo AM, Velly AM. A clinical and microbiological evaluation of denture cleansers for geriatric patients in long-term care institutions. J Can Dent Assoc. 2002; 68:39–45.
29. Karaagaclioglu L, Can G, Yilmaz B, Ayhan N, Semiz O, Levent H. The adherence of Candida albicans to acrylic resin reinforced with different fibers. J Mater Sci Mater Med. 2008; 19:959–963.
30. Waltimo T, Tanner J, Vallittu P, Haapasalo M. Adherence of Candida albicans to the surface of polymethylmethacrylate--E glass fiber composite used in dentures. Int J Prosthodont. 1999; 12:83–86.
31. Morgan TD, Wilson M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J Appl Microbiol. 2001; 91:47–53.
32. Zhou L, Tong Z, Wu G, Feng Z, Bai S, Dong Y, Ni L, Zhao Y. Parylene coating hinders Candida albicans adhesion to silicone elastomers and denture bases resin. Arch Oral Biol. 2010; 55:401–409.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download