Abstract
PURPOSE
The purpose of this study was to compare the fracture strength of the metal and the bond strength in metal-ceramic restorations produced by selective laser sintering (SLS) and by conventional casting (CAST).
MATERIALS AND METHODS
Non-precious alloy (StarLoy C, DeguDent, Hanau, Germany) was used in CAST group and metal powder (SP2, EOS GmbH, Munich, Germany) in SLS group. Metal specimens in the form of sheets (25.0 × 3.0 × 0.5 mm) were produced in accordance with ISO 9693:1999 standards (n=30). To measure the bond strength, ceramic was fired on a metal specimen and then three-point bending test was performed. In addition, the metal fracture strength was measured by continuing the application of the load. The values were statistically analyzed by performing independent t-tests (α=.05).
RESULTS
The mean bond strength of the SLS group (50.60 MPa) was higher than that of the CAST group (46.29 MPa), but there was no statistically significant difference. The metal fracture strength of the SLS group (1087.2 MPa) was lower than that of the CAST group (2399.1 MPa), and this difference was statistically significant.
Recently, rapid prototyping (RP) using 3-D images has been developed and its use has spread widely in the field of dental technology. RP is a common name for a variety of technologies1 for directly producing 3-D shaped products from computer aided design (CAD) files or digitally scanned data. Depending on the manner of production, RP technologies can be divided into stereolithography (SLA),2 laminated object manufacturing (LOM),3 selective laser sintering (SLS),4,5 and other technologies. In particular, SLS involves melting and laminating a metal powder with a laser on the basis of modeled CAD data.6 This technology has the advantage of shortening the production process and reducing the number of working hours, because the metal restoration can be produced in a digital environment without going through the complex process of wax carving, investment, and casting.7,8 Another advantage of this method is that less material is wasted than in the cutting method using a milling device, because lamination is carried out only on the necessary part. SLS technology is used widely in other industries,9 but it has only recently been introduced into the dental field for production of dental restorations.
Therefore, despite these advantages, there is a lack of data establishing the adequacy of these technologies for clinical applications, because very few studies assessing the quality of restorations produced by SLS have been carried out. The criteria for the clinical application of a new technology for producing restorations include biocompatibility of the materials,10 aesthetics,11 fracture resistance,12 corrosion resistance,13 and economic feasibility.14 In the case of metal-ceramic restorations specifically, the strength of the bond with the ceramic15,16 can also be an important criterion.
To meet these requirements, one-sided metal substructures can be produced using a centrifugal casting machine after the metal ingot is completely melted, as in conventional casting.17 However, SLS locally sinters powder particles in specific areas based on the amount of energy per unit area supplied by the irradiating laser. If the powder is only partially sintered by the laser, spaces will be present between particles because of the balling phenomenon18,19 and will affect both the strength of the metal itself and, especially, the strength of the bond between the metal and the ceramic. The balling phenomenon, which occurs typically in SLS, results in the combination of only a part of the center of a spherical particle with the nearby material.20,21 This phenomenon is affected by both the laser power and the scan speed.22,23,24 Therefore, the scan speed, particle size, specifications of the laser, and so on in the SLS process may affect the results. However, in some studies,25,26 the exact mechanism and specifications of the equipment used are not reported. Moreover, while differences in the bond strengths between the metal and ceramic have been reported, the strength values of the metal substructure and their dependence on the SLS conditions have not been reported.
Even though the bond strength between the metal and the ceramic is a very important factor determining whether the metal-ceramic restoration can be successfully produced by SLS, there have been very few reports on this until recently. Therefore, in this study, we have attempted to explore the clinical applicability of the SLS method by comparing the bond strength between the metal and ceramic and the fracture strength of the metal itself in metal-ceramic restorations produced using the conventional casting (CAST) method and SLS. The null hypothesis in this study is that the bond strength between the metal and the ceramic does not vary with the production method.
A non-precious alloy (StarLoy® C, DeguDent GmbH, Hanau, Germany) was used to produce the metal specimens used for CAST group, and Co-Cr metal powder (SP2; EOS GmbH, Munich, Germany) was used to produce the SLS group specimens. The composition of each material is presented in Table 1.
The coefficients of thermal expansion (CTEs) of the materials used in this study are shown in Table 2. Since a compressive stress occurs in the ceramic portion while the metal and ceramic cool after firing, the CTE of the metal must be slightly higher.27 Therefore, in this study, a ceramic powder (Vita VM13, Vita Zahnfabrik, Bad Säckingen, Germany) that fulfills these conditions was chosen.
To measure the bond strength of the metal and the ceramic, 15 metal specimens were produced for each group. The specimens were produced as sheets (25.0 × 3.0 × 0.5 mm) in accordance with the ISO 9693:1999 standards. To fabricate the SLS group specimens, designs were produced in CAD and converted to STL files. The files were transmitted to the SLS equipment (EOSINT M280, EOS GmbH, Munich, Germany), after which the Co-Cr powder was sintered by the laser. The specifications of this SLS method are based on the standard method recommended by the manufacturer. The scan speed was set to 7 m/s, the lamination thickness was set at 100 µm, and the Yb-fiber laser power was 200 W. The production speed was 20 m3/s, and the particle size of the metal powder was 20 µm.
However, to produce the metal specimens using the usual casting method (i.e., to produce the CAST group specimens), the mold of one of the SLS group specimens was replicated with silicone (Deguform® Plus, DeguDent Dentsply, Hanau, Germany). The wax pattern of this mold was produced by filling the mold with melted wax. The wax pattern was then invested with a phosphate-bonded investment for nonprecious metals, while the Co-Cr specimens were produced by centrifugation. The investment material and excess oxide layer attached to the surfaces of the specimens were sandblasted with 50 µm aluminum oxide (Al2O3) particles (Cobra, Renfert GmbH, Hilzingen, Germany) at a pressure of 3-4 bars. Sandblasting was also carried out for the SLS group specimens.
In order to observe the microstructure of the metal surface, one specimen was randomly selected from each group and observed under a scanning electron microscope (SEM). An accelerating voltage of 10.0 kV was employed. The observation area was limited to the portion that was to be fired to form the ceramic bond.
Ceramic samples of 8 mm in width, 3 mm in length, and 1 mm in height were fired on metal specimens of both the CAST and SLS group specimens according to the ISO 9693:1999 standards. In accordance with the instructions of the manufacturer, a thin opaque lining was applied before the ceramic was fired, and then the body ceramic was layered and fired.
To measure the metal-ceramic bond strength in each group, three-point bending tests were carried out with a universal testing machine (OTU-05D, Oriental TM Corp., Gyeonggi-do, Korea) in accordance with ISO Standard 9693.28 After the specimen was placed on a 20 mm wide support, a load was applied at a crosshead speed of 1.5 mm/min using a sphere of 1 mm in diameter. During the test, the fired ceramic portion was oriented toward the bottom. To evaluate the bond strength, the load at which the metal and ceramic failed was measured. Then, to measure the fracture strength of the metal itself, the load was continually applied even after the failure of the ceramic. To compare and analyze the measured values, a statistical program (SPSS 12.0, SPSS Inc., Chicago, IL, USA) was used and the results of each group were tested using independent t-tests at the significance level α=0.05.
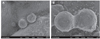
The properties of the ceramic-contacting surfaces of the metal specimens observed before firing were as follows. Diagonal lamination was observed on the surfaces of metal specimens produced by SLS (Fig. 1A and Fig. 1B). The thickness of each layer was about 100 µm, and the spacing between the layers was about 10-20 µm (black arrow)(Fig. 1C). However, the laminated structure was absent in the metal specimens in the CAST group (Fig. 1D and Fig. 1E). Furthermore, when the image of the surface of the SLS group specimen was magnified, the balling phenomenon was observed, resulting in spaces between two particles or locations where two particles touch each other (Fig. 2A and Fig. 2B).
The means and standard deviations of the bond strengths exhibited by the two groups of specimens are listed in Table 3. The mean (SD) bond strength of the SLS group specimens was 50.60 (6.27) MPa, and the mean (SD) bond strength of the CAST group specimens was 46.29 (5.96) MPa. These values indicate the absence of any statistically significant difference between the two groups. The mean (SD) fracture strength of the SLS group specimens was 1087.2 (112.8) MPa, and upon fracturing, the metal specimens separated into two pieces. However, when a load of 2399.1 (48.7) MPa was applied, the CAST group specimens were merely bent without fracture. The independent t-test result showed statistically significant difference between the two groups (Table 4).
For a valid comparison of the bond strengths between the metal and the ceramic obtained by using two different production methods, it is desirable that each ingredient used in the experiments should be identical,29 including the oxide film formation behavior,30 CTEs,31 etc. To minimize the effect of different composition and to find pure effect of manufacturing methods, materials with the most similar composition were chosen among materials provided (Table 1). In order to reduce the errors caused by the differences in the oxide film, the sandblasting times were set to be identical. To reduce the differences in the CTE, metals and ceramics with similar CTEs were used (Table 2).
The bond strengths showed no statistically significant difference, but it is estimated that the SLS group showed higher bond strengths than the CAST group because the gap caused by the additive manufacturing method widened the surface area. As shown in Fig. 1, the SLS specimens exhibited 100-µm-thick layers aligned in the laser irradiation direction. The maximum gap between the layers was around 20 µm, and the bond strength was likely to be increased because of the penetration of the ceramic powder through the gaps. Furthermore, according to Gu and Shen,32 the balling phenomenon occurs during layer-by-layer lamination during laser sintering. The balls formed at this time can be expected to increase the bond strength with the ceramic by increasing the surface area and by causing undercut. The studies performed by Korkmaz and Asar33 indicated that the bond strength between a Co-Cr alloy and a ceramic was 41.46-85.16 MPa, while Nieva et al.34 reported values of 57.11-63.81 MPa. These values are similar to or slightly higher than that reported in this study. Moreover, the results reported in this study are significantly higher than the minimum bond strength (25 MPa) between the metal and the ceramic specified in ISO 9693. Hence, it may be possible to use SLS to produce metal-ceramic restorations.
However, as seen in the results section, when a load was applied to the SLS group specimens even after the failure of the metal-ceramic bond, the metal portion fractured at 1087.2 MPa. It has been previously reported35 that the maximum occlusal load that can be applied to the posterior part is 1,031 MPa, which determines the limit at which clinical applications are possible. Furthermore, in the study undertaken by Fischer et al.,36 the experimental results for the Co-Cr alloy frame produced using the SLS method indicated a pattern similar to that observed in the present study, which further supports the clinical applicability of this method.
Based on the present study, SLS method showed similar bond strength to the conventional method. However, from a clinical point of view, this would have various advantages such as reduced working time, minimal material loss and minimal laboratory error.
In summary, according to the results of the present study, there was no significant difference between the metal-ceramic bond strengths obtained using SLS and casting. However, because of the layering produced by SLS, the fracture strength of the metal by itself appeared to be lower than that in the specimens produced by casting. Therefore, to increase the fracture strength of the metal itself while maintaining the metal-ceramic bond strength, additional studies are required to improve the materials and equipment used in SLS.
Figures and Tables
 | Fig. 1SEM images of SLS specimens magnified at ×33 (A), ×100 (B), and ×500 (C) and of CAST specimens at ×33 (D) and ×100 (E). The black arrow was the spacing between the layers (about 10-20 µm). |
Table 1
Material composition in wt%

| Co | Cr | W | Nb | V | Mo | Si | Fe | Mn | |
|---|---|---|---|---|---|---|---|---|---|
| SLS (SP2) | 61.8-65.8 | 23.7-25.7 | 4.9-5.9 | - | - | 4.6 5.6 | 0.8 1.2 | max 0.5 | max 0.1 |
| CAST (StarLoy® C) | 54.9 | 24.5 | 10.0 | 2.0 | 2.0 | 1.0 | 1.0 | 0.1 | - |
Table 2
CTEs of the materials used in the present study

| Physical characteristics | CTE (m/m℃) × 10-6 |
|---|---|
| SP2 | 14.0-14.5 |
| StarLoy® C | 14.0-14.3 |
| VITA VM13 | 13.1-13.6 (opaque) |
| 13.6-14.0 (base dentin) |
ACKNOWLEDGEMENTS
The authors would like to thank E-Master Dental Hub Laboratory for processing the Co-Cr specimens with the EOS equipment.
References
1. Traini T, Mangano C, Sammons RL, Mangano F, Macchi A, Piattelli A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent Mater. 2008; 24:1525–1533.
2. Hull CW. Apparatus for production of three-dimensional objects by stereolithography. US Patent. US4575330 A. 1984.
3. Feygin M, Shkolnik A, Diamond MN, Dvorskiy E. Laminated object manufacturing system. Torrance, CA: Helisys, Inc.;1998.
4. Deckard CR. Part generation by layerwise selective sintering. Austin, TX; USA: University of Texas at Austin;1986. Thesis (M.S. in Engin.).
5. Feygin M. Apparatus and method for forming an integral object from laminations. US Patent. EP0272305 B1. 1988.
6. Olakanmi EO. Selective laser sintering/melting (SLS/SLM) of pure Al, Al-Mg, and Al-Si powders: Effect of processing conditions and powder properties. J Mater Process Technol. 2013; 213:1387–1405.
7. van Noort R. The future of dental devices is digital. Dent Mater. 2012; 28:3–12.
8. Khaing MW, Fuh JYH, Lu L. Direct metal laser sintering for rapid tooling: processing and characterisation of EOS parts. J Mater Process Technol. 2001; 113:269–272.
9. Bremen S, Meiners W, Diatlov A. Selective Laser Melting. Laser Tech J. 2012; 9:33–38.
10. Wataha JC. Predicting clinical biological responses to dental materials. Dent Mater. 2012; 28:23–40.
11. Friebel M, Pernell O, Cappius HJ, Helfmann J, Meinke MC. Simulation of color perception of layered dental composites using optical properties to evaluate the benefit of esthetic layer preparation technique. Dent Mater. 2012; 28:424–432.
12. Ucar Y, Brantley WA, Johnston WM, Dasgupta T. Mechanical properties, fracture surface characterization, and microstructural analysis of six noble dental casting alloys. J Prosthet Dent. 2011; 105:394–402.
13. Manaranche C, Hornberger H. A proposal for the classification of dental alloys according to their resistance to corrosion. Dent Mater. 2007; 23:1428–1437.
14. Sturdevant JR, Sturdevant CM, Taylor DF, Bayne SC. The 8-year clinical performance of 15 low-gold casting alloys. Dent Mater. 1987; 3:347–352.
15. de Melo RM, Travassos AC, Neisser MP. Shear bond strengths of a ceramic system to alternative metal alloys. J Prosthet Dent. 2005; 93:64–69.
16. Salazar M SM, Pereira SM, Ccahuana V VZ, Passos SP, Vanderlei AD, Pavanelli CA, Bottino MA. Shear bond strength between metal alloy and a ceramic system, submitted to different thermocycling immersion times. Acta Odontol Latinoam. 2007; 20:97–102.
17. Watanabe K, Miyakawa O, Takada Y, Okuno O, Okabe T. Casting behavior of titanium alloys in a centrifugal casting machine. Biomaterials. 2003; 24:1737–1743.
18. Childs THC, Hauser C, Badrossamay M. Selective laser sintering (melting) of stainless and tool steel powders: Experiments and modelling. Proc Inst Mech Eng Part B. 2005; 219:339–357.
19. Gu D, Shen Y. Balling phenomena in direct laser sintering of stainless steel powder: Metallurgical mechanisms and control methods. Mater Des. 2009; 30:2903–2910.
20. Gusarov AV, Laoui T, Froyen L, Titov VI. Contact thermal conductivity of a powder bed in selective laser sintering. Int J Heat Mass Transf. 2003; 46:1103–1109.
21. Gu D, Shen Y. Effects of dispersion technique and component ratio on densification and microstructure of multi-component Cu-based metal powder in direct laser sintering. J Mater Process Technol. 2007; 182:564–573.
22. Li R, Liu J, Shi Y, Wang L. Balling behavior of stainless steel and nickel powder during selective laser melting process. Int J Adv Manuf Technol. 2012; 59:1025–1035.
23. Basile N, Gonon M, Petit F, Cambier F. Interaction between laser beam and BaTiO3 powders in selective laser sintering treatments. J Eur Ceram Soc. 2012; 32:3303–3311.
24. Su X, Yang Y. Research on track overlapping during selective laser melting of powders. J Mater Process Technol. 2012; 212:2074–2079.
25. Akova T, Ucar Y, Tukay A, Balkaya MC, Brantley WA. Comparison of the bond strength of laser-sintered and cast base metal dental alloys to porcelain. Dent Mater. 2008; 24:1400–1404.
26. Xiang N, Xin XZ, Chen J, Wei B. Metal-ceramic bond strength of Co-Cr alloy fabricated by selective laser melting. J Dent. 2012; 40:453–457.
27. Reyes MJ, Oshida Y, Andres CJ, Barco T, Hovijitra S, Brown D. Titanium-porcelain system. Part III: effects of surface modification on bond strengths. Biomed Mater Eng. 2001; 11:117–136.
28. ISO 9693. Metal-ceramic dental restorative systems. 2nd ed. Geneva, Switzerland: International Organization for Standardization;1999.
29. Kontonasaki E, Kantiranis N, Papadopoulou L, Chatzistavrou X, Kavouras P, Zorba T, Sivropoulou A, Chrissafis K, Paraskevopoulos KM, Koidis PT. Microstructural characterization and comparative evaluation of physical, mechanical and biological properties of three ceramics for metal-ceramic restorations. Dent Mater. 2008; 24:1362–1373.
30. Uusalo EK, Lassila VP, Yli-Urpo AU. Bonding of dental porcelain to ceramic-metal alloys. J Prosthet Dent. 1987; 57:26–29.
31. Zinelis S, Tsetsekou A, Papadopoulos T. Thermal expansion and microstructural analysis of experimental metal-ceramic titanium alloys. J Prosthet Dent. 2003; 90:332–338.
32. Gu D, Shen Y. Balling phenomena during direct laser sintering of multi-component Cu-based metal powder. J Alloys Compd. 2007; 432:163–166.
33. Korkmaz T, Asar V. Comparative evaluation of bond strength of various metal-ceramic restorations. Mater Des. 2009; 30:445–451.
34. Nieva N, Arreguez C, Carrizo RN, Molé CS, Lagarrigue GM. Bonding Strength Evaluation on metal/ceramic interfaces in dental materials. Proc Mater Sci. 2012; 1:475–482.
35. Esquivel-Upshaw JF, Anusavice KJ, Young H, Jones J, Gibbs C. Clinical performance of a lithia disilicate-based core ceramic for three-unit posterior FPDs. Int J Prosthodont. 2004; 17:469–475.
36. Fischer J, Stawarczyk B, Trottmann A, Hämmerle CHF. Festigkeit lasergesinterter Brückengerüste aus einer CoCr-Legierung. Quintessenz Zahntech. 2008; 34:140–149.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download