Abstract
PURPOSE
The purpose of this study was to evaluate bone response to anodized titanium implants coated with the extract of black cohosh, Asarum Sieboldii, and pharbitis semen.
MATERIALS AND METHODS
Forty anodized titanium implants were prepared as follows: group 1 was for control; group 2 were implants soaked in a solution containing triterpenoids extracted from black cohosh for 24 hours; group 3 were implants soaked in a solution containing extracts of black cohosh and Asarum Sieboldii for 24 hours; group 4 were implants soaked in a solution containing extracts of pharbitis semen for 24 hours. The implants from these groups were randomly and surgically implanted into the tibiae of ten rabbits. After 1, 2, and 4 weeks of healing, the nondecalcified ground sections were subjected to histological observation, and the percentage of bone-to-implant contact (BIC%) was calculated.
RESULTS
All groups exhibited good bone healing with the bone tissue in direct contact with the surface of the implant. Group 2 (52.44 ± 10.98, 25.54 ± 5.56) showed a significantly greater BIC% compared to that of group 3 (45.34 ± 5.00, 22.24 ± 2.20) with respect to the four consecutive threads and total length, respectively. The BIC% of group 1 (25.22 ± 6.00) was significantly greater than that of group 3 (22.24 ± 2.20) only for total length.
CONCLUSION
This study did not show any remarkable effects of the extract of black coshosh and the other natural products on osseointegration of anodized titanium implants as coating agents. Further studies about the application method of the natural products on to the surface of implants are required.
After the Brånemark group introduced osseointegrated titanium implants,1 research in this field has made remarkable progress. Even though dental implants have been very successful,2 titanium implants have an inherent limitation associated with their osteoinductive capacity to induce bone apposition. As a result, numerous studies have resulted in an increased titanium surface to obtain earlier and more firm osseointegration.3,4 To improve titanium implant surfaces, extensive research has focused on the addition of bioactive agents, recently.5 Several categories of bioactive agents have been used for implant surface coating. Bioceramics such as calcium phosphate ceramics (e.g., hydroxyapatite), bioactive proteins including bone morphogenetic proteins (BMPs), collagen, and peptides have been studied.6 Fluoride ions and polymers (e.g., chitosan) have also been investigated. Theoretically, these bioactive implants have the advantage of rapid biochemical attachment prior to the development of proper biomechanical bonding. Albrektsson and Wennerberg3 reported that although the existence of bioactivity has yet to be proven, several indications for biochemical bonding have been presented in the scientific literature.
Since natural products are good medicinal reagents based on their stability, low occurrence of side effects, and diverseness, the use of and search for drugs and dietary supplements derived from natural products have been accelerated.7,8 Recently, several natural products have been evaluated for their effects on the bone metabolism associated with osteoporosis and periodontal disease.9-14
Black cohosh is the common name of the root and rhizoma sourced from Cimicifuga racemosa of the Ranunculaceae family. It has long been used as an alternative for estrogen replacement therapy in modern medicine to treat climacteric complaints that include hot flashes and osteoporosis in North America and Europe.15 It has been determined that the main constituents of the rhizoma are highly oxidized cycloartane-type terpenoids and phenol-type derivates. Based on research findings, triterpenoids, which are known to be an active derivate of black cohosh, have been shown to exert an inhibitory effect on osteoclastic bone resorption through the suppression of both the formation and activity of osteoclastic-like cells in vitro and have shown a significant protective effect on bone mineral density when systemically administered in vivo.16 The extract of black cohosh (EBC), one of the top ten natural products sold in the United States and widely used in Europe for reducing climacteric symptoms including osteoporosis and hot flashes, gained attention in the field of bone research because of strong evidence of their osteoprotective effects.10,15 Li et al.16 reported inhibitory activities of some cycloartane-type triterpenoids isolated from EBC on bone resorption through the suppression of both the formation of osteoclast-like cells and their resorbing activities in vitro. However, despite many studies attempting to explain the mechanism of the extract, this topic is still unresolved. Summarizing the evidence, it is theorized that the effects of EBC might be mediated by unknown estrogen receptors or unidentified estrogenic substances not involving estrogen receptors.10 Considering the research showing that estrogen had direct anabolic effects on osteoblasts in vitro,17 the mechanism of the direct effect of EBC on bone cells could be related to the estrogen-like actions of EBC. Investigations about the effects of the extract of pharbitis semen (EPS) on bone have recently begun.18 It was found that EPS inhibited the formation of nitric oxide, suggesting that it would promote the activity of osteoblasts based on previous studies about the inverse relationship between the concentration of nitric oxide and the activity of osteoblasts.19 In addition, the activity of alkaline phosphatase, an indicator of osteoblast differentiation, increased when osteoblast cells had been treated with EPS. However, the method through which EPS inhibits the formation of nitric oxide is still being investigated. In vivo, In recent years, phytochemical studies on EPS have resulted in the isolation of various terpenoid groups.20,21 As steroids and sterols in animals are biologically produced from terpenoid precursors, active derivatives of EPS that affect bone cells are expected to be discovered in the near future. Various efforts to effectively deliver the drug to the surgical site have been made.
Asarum sieboldii has been used to clinically treat the common cold because of its effects on rhinitis, allergic disease, and sinusitis.20 There have been several reports on the isolation of methyleugenol, safrole, eucarvone, elemicin, asarinin, and asarone from Asarum sieboldii. It has been shown that the main constituents, safrole and asaricin, have antimicrobial and anti-inflammatory effects.18,22 Since infections on and around the titanium implant surface are usually difficult to treat and may result in implant removal, antibacterial coatings on dental implants have been used to inhibit the initial attachment of bacteria to the titanium.23 Many kinds of substances such as antibiotics, non-antibiotic organics, and inorganic antimicrobial agents have been proposed for use in this strategy.24-26 Pharbitis nil choisy is a short-day plant that is distributed throughout Southeast Asia. The extract of pharbitis semen, the seed of this plant, has been used in folkloric medicine.27 Previous phytochemical studies on the seeds of Pharbitis nil choisy have resulted in the isolation of resin glycosides, gibberellins, flavonoids, diterpene glycosides, and triterpenoid saponins.27,28 Research about the effect of the extract of pharbitis semen on bone has been recently conducted in an attempt to investigate its pharmaceutical composition for use in the prevention or treatment of periodontal disease. Researchers found that the extract promoted the differentiation and mineralization of osteoblasts in vitro by inhibiting the formation of nitric oxide and by increasing the level of alkaline phosphatase activity.28 Baek12 also reported an osteogenetic effect of the pharbitis semen extract in a rabbit calvarial model.
This study attempted to verify the potential of the black cohosh which showed osteogenetic effects previously, as a bioactive agent coated onto the dental implant. And Asasrum sieboldii was added to black cohosh expecting synergistic anti-inflammatory effect.
Threaded implants were manufactured via the machining of commercially pure titanium (Grade 4) (Warentec Co., Seoul, Korea). The implants had lengths of 7.0 mm, outer diameters of 3.75 mm, and pitch-heights of 0.6 mm. The anodic oxidation treatment of the implant was performed at 300 V in an aqueous electrolytic solution of 0.02 M calcium glycerophosphate and 0.15 M calcium acetate. All procedures were performed at room temperature in a total single implant anodization time of three minutes.29,30 A total of 40 implants were washed with distilled water, dried, and sterilized in ethylene oxide (EO) gas prior to animal surgery.
Black cohosh (Cimicifugae rhizoma), Asarum sieboldi, and pharbitis semen were purchased from Geumgang Pharmaceuticals Corp (Masan, Korea). The specimen identifications were confirmed at the Lab of Oriental Medicinal Cosmetic Pharmacology at Kyung-Hee University in Seoul, Korea. The extraction of black cohosh with decoction was performed as follows: 150 g of finely powdered material from the dried black cohosh was soaked in 1.5 L of water (1 : 10 wt = vol) and boiled for 2 hours. The extract was then filtered through Whatman No. 1 filter paper (Whatman Ltd., Maidstone, UK) and evaporated under vacuum at 40℃ using a rotary evaporator (EYELA, Tokyo, Japan). The decocted extract yielded 12 g of powdered material. The extraction of the mixture of Asarum sieboldii and black cohosh was prepared through the same method. One hundred forty grams of Asarum sieboldii and black cohosh (1 : 6 weight ratio) were processed to prepare 15 g of decocted extract. The extraction of pharbitis semen (seed of Pharbitis nil choisy) with ethanol was performed as follows: 2 kg of finely powdered dried pharbitis semen was soaked in ethanol (1 : 10 wt = vol) for seven days at room temperature. It was then filtered through Whatman No. 1 filter paper and evaporated under vacuum at 40℃. The ethanol extract of pharbitis semen yielded 48.5 g of powdered material after vacuum evaporation.12,18
The implant samples were divided into four groups. Group 1 was the control, while groups 2, 3, and 4 were soaked in distilled water containing the extracts of medicinal plants (0.5 mg/10 mL) for 24 hours.31,32
Group 1: Control
Group 2: Decocted extract of black cohosh
Group 3: Decocted extracts of black cohosh and Asasarum sieboldii
Group 4: Decocted extracts of pharbitis semen
This animal experiment was approved by the Institutional Animal Care and Use Committee in the Asan Institute of Life Science (No. 200802105) and followed the guidelines of the Laboratory of Animal Research of Asan Medical Center in Seoul, Korea. The Ten New Zealand white rabbits, each weighing 3 to 3.5 kg, were used in this study. The animals were housed in separate cages and fed a standard diet. For surgery, general anesthesia was induced through the intramuscular injection of 10 mg/kg of Zoletil (Vibac, Carros Cedex, France) and 0.15 mL/kg of Rompun (Bayer Korea, Ansan, Korea). Both rear legs of each rabbit were shaved and washed with iodine solution. Two percent lidocaine (Yu-han, 1.0 mL, epinephrine 1:100,000) was administered at the tibial area. Using sterile surgical techniques, an incision was made into the skin to expose the proximal aspect of each tibia. The muscles were then dissected to allow for the elevation of the periosteum. The flat surface on the lateral aspect of the proximal tibia was selected for implant placement. Holes were drilled into the tibia with a low-speed rotary instrument under constant irrigation with sterile saline. Each rabbit had two implants inserted into each tibia. A total of 40 implants were inserted in a randomized 4 × 4 latin square design that enabled multiple comparisons (Fig. 1). Muscle and fascial layers were closed in layers with Vicryl resorbable sutures (Woori Medical, Namyangju, Korea), while the skin was sutured with black silk for primary closure. Postoperatively, all animals received 50 mg/kg of Cefazolin sodium (Chong Kun Dang Pharm., Seoul, Korea) intramuscularly.33
Two rabbits after one week, four rabbits after two weeks and the remaining four rabbits after four weeks were sacrificed for histological evaluation.34,35 The implants and the surrounding bone were harvested en bloc and fixed in neutral buffered formalin, dehydrated in 70%, 90%, 95%, and 100% alcohol, and embedded in a light-curing resin (Technovit 7200 VLC; Kulzer, Wehrheim, Germany). An Exakt sawing machine with grinding equipment (Exakt Apparatebau, Norderstedt, Germany) was used to cut and grind sections approximately 50 um thick that were then stained with 1% toluidine blue prior to evaluation under a light microscope.36 All animals underwent histologic examination with the aid of an Olympus BX microscope (Olympus, Tokyo, Japan) connected to a computer. Image Tool Ver. 3.0 (San Antonio Dental School, University of Texas Health Science Center, USA) software was used to calculate the extent of bone-to-implant contact (BIC%) in the four consecutive threads and in the total length of the implant.37 All the measurements were calculated under 100×magnification. A higher magnification objective and zoom were used to help determine whether or not the bone was in contact with the implant surface. The histomorphometric analysis was conducted for the animals that were sacrificed after two and four weeks of healing, while those sacrificed after one week were only histologically observed (Fig. 2).
A mixed model analysis was performed to evaluate the histomorphometric measurment using statistical R software. Differences among the groups were examined using the Bonferroni correction. The level of statistical significance was set at 5%.
After healing periods of one, two, and four weeks, all of the implants were stable without any complications. After one week, endosteal bone surfaces showed signs of bone formation via appositional growth which sometimes reached the implant surface in all groups (Fig. 3). No gaps or connective tissue at the bone-implant interface were observed in any of the groups. An increased amount of newly formed bone filling the threads and a compaction of the bone were characteristic features observed after two weeks of healing (Fig. 4). After four weeks, new bone in all groups showed signs of remodeling in locations at which woven bone had been replaced with lamellar bone (Fig. 4).
The BIC% in the four consecutive threads and the BIC% in the total length were calculated after two and four weeks (Table 1). After two weeks, the mean BIC% of group 2 demonstrated greater mean values compared to those of the other groups when measuring all threads around the implants as well as the four best consecutive threads, but no significant differences were seen. After four weeks, groups 2 and 4 showed similar mean BIC%s to that of the control group, while group 3 showed a smaller mean BIC%. Considering the results from the mixed model analysis, there was no significant random effect for each rabbit with respect to differences in BIC%. Therefore, it was determined that all differences in BIC% were due to the surface treatments. Fig. 5 shows comparisons of the bony contacts in the four consecutive threads and in all of the threads of each group, respectively. Group 2 showed a significantly greater BIC% compared with that of group 3 with respect to the four consecutive threads and total length. The BIC% of group 1 was significantly greater than that of group 3 with regard to the total length.
In the area of dentistry, a number of these natural products have been recently investigated for their general antimicrobial activities for preventing oral disease.38 The aim of the present study was to evaluate the extract of natural products that have shown osteogenetic effects as bioactive agents coated onto the surface of titanium implants. In the histological observations in this study, all groups illustrated good bone healing, showingbone tissue in direct contact with the titanium implant surface. However, there were no experimental groups which exhibited significantly superior bone response compared to that of the control group. Specimens treated with Asarum sieboldii that has antimicrobial property and black cohosh (group 3) showed significantly lower BIC%s than those of the other experimental and control groups. This might be caused by drug - Asarum sieboldii interaction. The metabolic result of black cohosh from Asarum sieboldii was not taken into consideration in this study and that might be the reason for low BIC in group 3. Group 2 and 4 did not show significant effect compared to control group. The concentration of natural products was decided based on previous studies,15 but that concentration might not be sufficient enough for this study design. Further studies are needed for this matter.
EBC and EPS showed potential as bioactive agents because they exhibited good bone healing with the implants in this study and in previous studies.12 Although EBC and EPS showed good results in previous in vivo and in vitro studies, no experimental groups exhibited significantly superior bone response compared to that of the control group. Presumably, this lack of difference is due to a possible cause. The method in which the extracts were delivered to the surgical sites might not have been an effective system with anodized titanium implants soaked in extract solutions for 24 hours. The pores in the anodized oxide layer were the result of micro arc anodes. These pores were used as drug delivery systems for bioactive agents involved in biochemical attachment in the study. Scaffolds such as PLGA, canals on the implant structure, and micro pores caused by titanium surface treatments have been suggested.31,39-40 Further studies about the drug delivery system which delivers EBC and EPS to the surgical sites and allows them continue to have an effect on osteogenesis may result in positive findings associated with osseointegration.
Developmentally, two weeks in the life of a rabbit are equivalent to one and a half months in the life of a human. This equates to an approximately three times more rapid healing process in rabbits compared to that of humans.41 In the rabbit tibia model in this study, traditional implant protocols recommended a rest period of at least three months prior to the application of load from masticatory forces to the implants and surrounding bone via prostheses. With this model, early bone responses were adequately evaluated in a relatively short period. In addition, we investigated characteristic of adverse reactions such as inflammation or rejection after a week of treatment with extract of black cohosh, pharbitis semen or Asarum sieboldii as bioactive coating materials on implant surfaces. Although the study did not show remarkable effect of the extracts of black coshosh and the other natural products on osseointegration of anodized titanium implants, further studies about the application method on to the surface of implants are needed.
In this study, the triterpenoids extracted from black cohosh did not play superior role in osseointegration. And other extracts of the natural products did not have significant effect either. Further studies about an effective transport system of the extracts of natural products and a method for retaining the osteogenetic effects for a longer period may lead to positive results on osseointegration.
Figures and Tables
 | Fig. 3Light microscope view (toluidine blue; original magnification ×100) at 1 week: (A) control group; (B) group 2; (C) group 3; (D) group 4. Endosteal bone surfaces showed signs of bone formation (arrow) by appositional growth which sometimes reached the implant surface in all groups. |
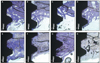 | Fig. 4Light microscope views (toluidine blue; original magnification ×100) at two weeks: (A) control group; (B) group 2; (C) group 3; (D) group 4. And at four weeks: (E) control group; (F) group 2; (G) group 3; (H) group 4. |
 | Fig. 5(A) The percentages of bone-to-implant contact in the four consecutive threads. Group 2 showed a significantly greater BIC% than did group 3 (P<.05). (B) Groups 1 and 2 showed significantly greater BIC%s than did group 3 with respect to total length (P<.05). |
Table 1
Percentage of bone-to-implant contact

References
1. Brånemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969; 3:81–100.
2. Ostman PO. Immediate/early loading of dental implants. Clinical documentation and presentation of a treatment concept. Periodontol 2000. 2008; 47:90–112.
3. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004; 17:536–543.
4. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 2-review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004; 17:544–564.
5. Junker R, Dimakis A, Thoneick M, Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 2009; 20:185–206.
6. Avila G, Misch K, Galindo-Moreno P, Wang HL. Implant surface treatment using biomimetic agents. Implant Dent. 2009; 18:17–26.
7. Gullo VP, McAlpine J, Lam KS, Baker D, Petersen F. Drug discovery from natural products. J Ind Microbiol Biotechnol. 2006; 33:523–531.
8. Lee KH. Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J Nat Prod. 2010; 73:500–516.
9. Putnam SE, Scutt AM, Bicknell K, Priestley CM, Williamson EM. Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother Res. 2007; 21:99–112.
10. Li JX, Yu ZY. Cimicifugae rhizoma: from origins, bioactive constituents to clinical outcomes. Curr Med Chem. 2006; 13:2927–2951.
11. Kim HY, Kim CS, Jhon GJ, Moon IS, Choi SH, Cho KS, Chai JK, Kim CK. The effect of safflower seed extract on periodontal healing of 1-wall intrabony defects in beagle dogs. J Periodontol. 2002; 73:1457–1466.
12. Baek EJ. The extract of Pharbitis nil Choisy has bone regenerative potential in the healing process of calvarial defects in a rabbit model. Seoul: Kyunghee Univ;2009. Master's Thesis.
13. Chen LL, Tang Q, Yan J. Therapeutic effect of aqueous-extract from a traditional Chinese medical herb Drynaria fortunei on rat experimental model of alveolar bone resorption. Zhongguo Zhong Yao Za Zhi. 2004; 29:549–553.
14. Kesavalu L, Vasudevan B, Raghu B, Browning E, Dawson D, Novak JM, Correll MC, Steffen MJ, Bhattacharya A, Fernandes G, Ebersole JL. Omega-3 fatty acid effect on alveolar bone loss in rats. J Dent Res. 2006; 85:648–652.
15. Wuttke W, Gorkow C, Seidlová-Wuttke D. Effects of black cohosh (Cimicifuga racemosa) on bone turnover, vaginal mucosa, and various blood parameters in postmenopausal women: a double-blind, placebo-controlled, and conjugated estrogens-controlled study. Menopause. 2006; 13:185–196.
16. Li JX, Liu J, He CC, Yu ZY, Du Y, Kadota S, Seto H. Triterpenoids from Cimicifugae rhizoma, a novel class of inhibitors on bone resorption and ovariectomy-induced bone loss. Maturitas. 2007; 58:59–69.
17. Ernst M, Heath JK, Schmid C, Froesch RE, Rodan GA. Evidence for a direct effect of estrogen on bone cells in vitro. J Steroid Biochem. 1989; 34:279–284.
18. Jung WS, Yoo HM, Seo SW, Cho JK, Son JW, Park MC, Choi CM, Yeon SR, Hwang SW, Kim YW, Song DS, Chae YS, Park SJ, Shin MK, Song HJ. Anti-inflammatory effect of extract of Asaum sieboldii in LPS-stimulated Murine peritoneal macrophage. Korean J Herbol. 2006; 21:189–195.
19. Riancho JA, Zarrabeitia MT, Fernandez-Luna JL, Gonzalez-Macias J. Mechanisms controlling nitric oxide synthesis in osteoblasts. Mol Cell Endocrinol. 1995; 107:87–92.
20. Hashimoto K, Yanagisawa T, Okui Y, Ikeya Y, Maruno M, Fujita T. Studies on anti-allergic components in the roots of Asiasarum sieboldi. Planta Med. 1994; 60:124–127.
21. Jung da Y, Ha H, Lee HY, Kim C, Lee JH, Bae K, Kim JS, Kang SS. Triterpenoid saponins from the seeds of Pharbitis nil. Chem Pharm Bull (Tokyo). 2008; 56:203–206.
22. Villegas M, Vargas D, Msonthi JD, Marston A, Hostettmann K. Isolation of the antifungal compounds falcarindiol and sarisan from Heteromorpha trifoliata. Planta Med. 1988; 54:36–37.
23. Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009; 91:470–480.
24. Alt V, Bitschnau A, Osterling J, Sewing A, Meyer C, Kraus R, Meissner SA, Wenisch S, Domann E, Schnettler R. The effects of combined gentamicin-hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials. 2006; 27:4627–4634.
25. Campbell AA, Song L, Li XS, Nelson BJ, Bottoni C, Brooks DE, DeJong ES. Development, characterization, and anti-microbial efficacy of hydroxyapatite-chlorhexidine coatings produced by surface-induced mineralization. J Biomed Mater Res. 2000; 53:400–407.
26. Chen W, Liu Y, Courtney HS, Bettenga M, Agrawal CM, Bumgardner JD, Ong JL. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials. 2006; 27:5512–5517.
27. Kim KH, Choi SU, Lee KR. Diterpene glycosides from the seeds of Pharbitis nil. J Nat Prod. 2009; 72:1121–1127.
28. Lee TH, Park YD, Lee KJ, Jung JH, Kang DH. Pharmaceutical composition for preventing or treating periodontal disease comprising extracts of Pharbitidis Semen 2008. Patent #1020080035877 from Korean Intellectual Property Office.
29. Li LH, Kong YM, Kim HW, Kim YW, Kim HE, Heo SJ, Koak JY. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004; 25:2867–2875.
30. Choi JW, Heo SJ, Koak JY, Kim SK, Lim YJ, Kim SH, Lee JB. Biological responses of anodized titanium implants under different current voltages. J Oral Rehabil. 2006; 33:889–897.
31. Park JM, Koak JY, Jang JH, Han CH, Kim SK, Heo SJ. Osseointegration of anodized titanium implants coated with fibroblast growth factor-fibronectin (FGF-FN) fusion protein. Int J Oral Maxillofac Implants. 2006; 21:859–866.
32. Lan J, Wang ZF, Shi B, Xia HB, Cheng XR. The influence of recombinant human BMP-2 on bone-implant osseointegration: biomechanical testing and histomorphometric analysis. Int J Oral Maxillofac Surg. 2007; 36:345–349.
33. Lee JE, Heo SJ, Koak JY, Kim SK, Han CH, Lee SJ. Healing response of cortical and cancellous bone around titanium implants. Int J Oral Maxillofac Implants. 2009; 24:655–662.
34. Yang GL, He FM, Yang XF, Wang XX, Zhao SF. Bone responses to titanium implants surface-roughened by sandblasted and double etched treatments in a rabbit model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:516–524.
35. Burgos PM, Rasmusson L, Meirelles L, Sennerby L. Early bone tissue responses to turned and oxidized implants in the rabbit tibia. Clin Implant Dent Relat Res. 2008; 10:181–190.
36. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982; 11:318–326.
37. Wennerberg A, Albrektsson T, Andersson B. Bone tissue response to commercially pure titanium implants blasted with fine and coarse particles of aluminum oxide. Int J Oral Maxillofac Implants. 1996; 11:38–45.
38. Palombo EA. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid Based Complement Alternat Med. 2011; 2011:680354.
39. Lee SY, Koak JY, Heo SJ, Kim SK, Lee SJ, Nam SY. Osseointegration of anodized titanium implants coated with poly(lactide-co-glycolide)/basic fibroblast growth factor by electrospray. Int J Oral Maxillofac Implants. 2010; 25:315–320.
40. Stenport VF, Johansson C, Heo SJ, Aspenberg P, Albrektsson T. Titanium implants and BMP-7 in bone: an experimental model in the rabbit. J Mater Sci Mater Med. 2003; 14:247–254.
41. Roberts WE, Smith RK, Zilberman Y, Mozsary PG, Smith RS. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod. 1984; 86:95–111.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download