Abstract
PURPOSE
The purpose of this study was to analyze the effect of denture cleansers on Candida albicans biofilm formation over resilient liners and to evaluate compatibility between resilient liners and denture cleansers.
MATERIALS AND METHODS
Acrylic resin (Lucitone 199®) and 3 resilient liners (COE-SOFT™, GC RELINE™ and SOFRELINER TOUGH TOUGH®) were incubated in denture cleansers (Polident® and Cleadent®) for 8 hours a day and in unstimulated saliva for 16 hours a day (n=25/gp) for 60 days. Two-way and three-way repeated measures ANOVA were performed to compare the surface roughness (Ra), pH and C. albicans binding level by radioisotope (α=0.05). The statistical significance of the relation between Ra and adhesion was evaluated by correlation analysis.
RESULTS
The degree of Ra was significantly decreased in the following order: COE-SOFT™, acrylic resin, GC RELINE™ and SOFRELINER TOUGH®. The immersion in denture cleansers significantly increased Ra of resilient liners, except for SOFRELINER TOUGH® in Cleadent®. No significant differences in pH curves were observed among groups immersed in distilled water and denture cleansers. The binding levels of C. albicans were significantly decreased in the following order: COE-SOFT™, GC RELINE™, SOFRELINER TOUGH®, and acrylic resin. The immersion in Cleadent® seemed to decrease C. albicans binding level on GC RELINE™ and SOFRELINER TOUGH®.
Denture stomatitis is an erythematous pathogenic condition of denture bearing mucosa, caused mainly by microbial factors, especially Candida albicans.1 Its prevalence has been reported at 11-67% in complete denture wearers.2 The main reservoir of C. albicans and related Candida species has been shown to be the tissue surface of maxillary complete dentures.1,3 Recently, it has been pointed out that continuous swallowing or aspiration of microorganisms from denture plaque can cause unexpected infections to immunocompromised host or medicated old person.4
Two methods have been proposed for routine denture biofilm removal including mechanical and chemical cleansing.5 The chemical method is considered to be the most effective for inhibiting C. albicans infection and denture biofilm formation.5,6,7 However, the routine use of denture cleansers has been known to cause adverse effects on physical characteristics of denture materials and resilient liners. Goll et al.8 reported that resilient liners increased discoloration, porosity, surface and size changes, and solubility by the use of denture cleanser for 30 days, Harrison et al.7 reported that resilient liners were damaged by alkaline peroxide type of denture cleanser, and Nikawa et al.9 reported that peroxide type of denture cleanser caused damage to resilient liners, and was not related to the quantity. There have been numerous studies to investigate compatibility between resilient liners and denture cleansers, primarily focusing on changes of physical properties such as surface roughness, viscoelastic properties, and color.7,8,9 However, there is not much information available about C. albicans biofilm formation on resilient liners which are immersed in denture cleansers within a given period.
Therefore, this study was aimed to analyze the effect of denture cleansers on C. albicans biofilm formation over resilient liners and to evaluate compatibility between resilient liners and denture cleansers. The null hypothesis was that there would be no significant differences in the degree of Ra, pH and the binding level of C. albicans among groups with immersing in different denture cleansers.
Three commercially available resilient liners were investigated, acrylic resin using as control group (Table 1). Each specimen was processed according to the manufacturer's directions and prepared to be a uniform size (14 mm diameter × 1 mm thickness) with a smooth surface by placing glass slides. Two commercial denture cleansers were studied using distilled water (DW) as control solution (Table 2).
Candida albicans ATCC 10231 was purchased from American Type culture collection and used in this study. C. albicans was cultured using Trypicase soy broth (TSB; Beckton Dickinson, Sparks, MD, USA) at 37℃ in aerobic condition. To investigate adhesion assay, the yeast was incubated with specimens in TSB aerobically at 37℃ for 18 hours.
Unstimulated whole saliva (UWS) was collected by spitting method from non-smoking healthy persons in glass bottle on ice. For saliva collection, the Institutional Review Board at Korea University Guro Hospital approved this study protocol (MD11006). Saliva was centrifugated at 6,500 × g for 5 min at 4℃, and then the supernatant was diluted to a ratio of 1:1 with phosphate buffered saline and filtered by polyvinylidene difluoride (PVDF) membrane with pore size of 0.22. Filtered-saliva was stored at 4℃.
The specimens was incubated serially in denture cleanser for 8 hours at room temperature, washed three times with autoclaved PBS and in UWS for 16 hours at 37℃. The soaking stage was repeatedly carried out every day for 60 days. Fifteen of seventy-five specimens were used for the measurements of average surface roughness (Ra), and the remainder was used for biofilm assay.
Denture cleanser-treated specimens were washed three times with distilled water and dried using airbrush until removing distilled water. The average surface roughness of specimen was measured three times with a profilometer (Surtronic 3P; Taylor-Hobson, Leicester, UK).
The cultured medium was centrifuged at 3,000 × g for 10 min, and the supernatant was placed to a new tube. The pH of the cultured medium was measured at indicated times (12, 24, 36, and 48 hours).
Radio-labeling was performed by anaerobically incubating C. albicans in 10 mL of TSB containing 1 µCi/mL methyl-[3H] thymidine (TSBH; Amersham Pharmacia Biotech, NJ, USA) for 18 hours at 37℃. In order to assay adhesion, 5 mL of the culture suspension was inoculated in 500 mL of TSBH, and then the TSBH containing the isotope-labeled C. albicans was dispensed into 85 mm-diameter dish.
After 15 samples of specimen were placed on 85 mm-diameter dish, 20 mL of TSBH which contained radiolabeled C. albicans was added. The dish was then incubated for 48 hours at 37℃, and the samples were washed three times with phosphate buffered saline for removing unbound C. albicans. In order to detach C. albicans, the dish was vortexed in 4 mL of lysis buffer [0.5 M Tris (pH 9.0), 10 mM NaCl, 20 mM EDTA, 1% SDS], and 3 mL of each the suspension was mixed with 3 mL of aqueous cocktail solution (Packard, CT, USA), and the radioactivity was counted with β-counter (BD, NJ, USA).
SPSS (Ver. 10.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The statistical significances were evaluated by two-way or three-way ANOVA, and Mann-whitney test. Every analysis was approved with 95% reliance. The statistical significance of the relation between Ra and adhesion was evaluated by correlation analysis.
The degree of Ra was decreased in the following order: COE-SOFT™, acr ylic resin, GC RELINE™ and SOFRELINER TOUGH® (P<.05, Table 3, Fig. 1). Ra values of acrylic resin and COE-SOFT™ immersed in denture cleanser, especially in Cleadent®, were higher than in D.W. Similarly, Ra values of polysiloxanes (GC RELINE and SOFRELINER TOUGH ) immersed in denture cleansers were higher than in D.W, except for SOFRELINER TOUGH® in Cleadent®.
The pH of each medium is shown in Fig. 2, and the growth curve of C. albicans in medium immersing resilient liners presented a reverse S-shape. The pH of medium decreased rapidly until 24 h, and slowly after 36 h and 48 h. The difference between each denture relining material and denture cleanser was not observed significantly between each denture relining material and denture cleanser was not observed (P>.05). Changes with time were shown to be significantly different (P<.01).
The binding levels of C. albicans were decreased in the following order: COE-SOFT™, GC RELINE™, SOFRELINER TOUGH®, and acrylic resin (P<.05, Table 4, Fig. 3). Biofilm formation on acrylic resin was greater when immersed in denture cleansers than in D.W. Also, biofilm formation on COE-SOFT™ was significantly increased in denture cleansers than in D.W.. Interestingly, however, biofilm formation on polysiloxanes was significantly increased in Polident®, however, decreased in Cleadent®.
The statistical significance of relationship between Ra and biofilm formation of C. albicans was evaluated with correlation analysis, however, there was only a very low interrelationship (r=0.184; Pearson correlation coefficients).
This study aimed to analyze the effect of denture cleansers on C. albicans biofilm formation over resilient liners by evaluating Ra, pH change, and C. albicans binding level. There are several reports to suggest the relationship between surface roughness and C. albicans adherence to denture materials.10,11,12 Verran et al.10 reported that significantly higher number of C. albicans was observed on roughened than on smooth surfaces, and Radford et al.12 reported significantly greater adhesion of C. albicans to rough than smooth surfaces. If repetitive immersion in denture cleanser roughens the surface of resilient liners, C. albicans adhesion would be expected to increase. Therefore, we evaluated Ra in this study, and presented the average surface roughness (Ra) of soft relining materials immersed in denture cleansers is shown in Table 1. PMMA (COE-SOFT™) showed the greatest, whereas polysiloxanes the least Ra. The plasticizer component of PMMA (for example, dibutylphthalate) was leached out in the thermocycling process more than polysiloxane, therefore, Ra of PMMA may have been increased. Acrylic resin and PMMA immersed in denture cleanser showed increased Ra.
This may be due to deterioration of PMMA components by denture cleansers during the thermocycling process, regardless of capacity of denture cleanser to remove C. albicans biofilm.
The major pathology of denture stomatitis is the growth and acid production of C. albicans on denture fitting surface.13 Acid production of C. albicans induces cytotoxin. Furthermore, C. albicans activates acid proteinase and phospholipase, and aggregates Candida.14 Nikawa et al.15 reported that resilient liners inhibits C. albicans growth, colonization and biofilm formation. The inhibitory effects of resilient liners on fungal growth were observed in the following three manners: delay in the beginning of rapid decline in pH, decreases in the rate of pH change and increases in minimum pH.16,17 An expected, therefore, we observed changes of medium pH by resilient liners when immersed in denture cleanser. The pH changes in culture medium varied according to resilient liners and denture cleansers, however statistically significant difference was not observed between groups immersed in D.W and denture cleansers for 60 days (Fig. 2, P>.05).
Goll et al.8 reported that the physical characteristics of soft relining materials are deteriorated by using denture cleanser for 30 days, and Harrison et al.7 and Nikawa et al.9 showed that peroxide type of denture cleansers damages soft relining materials. Moreover, Nikawa et al.13 reported that mismatched use of denture liners and denture cleanser increases biofilm formation. In the present study, however, daily use of denture cleansers did not always aggravate Candida biofilm formation on resilient liners. Although biofilm formation of C. albicans on acrylic resin and PMMA (COE-SOFT™) was increased by using denture cleansers, it was decreased on polysiloxanes (GC-Reline™ and SOFRELINER TOUGH®) by using Cleadent® (Fig. 3). The biofilm formation level of C. albicans on PMMA was the highest when two denture cleansers were used: Polident® and Cleadent®. Further researches are needed to investigate the biofilm formation level of C. albicans on other PMMA-based resilient liner. In polysiloxane groups, Cleadent® was more effective to remove biofilm of C. albicans than Polident®. Polident® consisted of sodium perborate, oxone and everase while Cleadent® consisted of oxybleach, proteinase, flabonoid, anion detergent, carbonate, and organic acid.
In this study, we evaluated the relationship between biofilm formation and Ra, and the result showed low correlation (r=0.184), thus implying that Ra of each specimen is not the essential factor to form biofilm of C. albicans. C. albicans biofilm formation was found to increase on polysiloxane groups when Polident® was used, and to decrease by using Cleadent®. As there is little overlap among ingredients which manufacturers indicated, it is not possible at present to find out which ingredient of denture cleansers caused this opposite result. Additional study is needed to find out which ingredient of cleansers has adverse effects on denture liners.
Figures and Tables
 | Fig. 1Average surface roughness (Ra) of acrylic resin and resilient liners immersed in denture cleansers (mm). Immersion in denture cleansers increased Ra of soft relining materials, except for SOFRELINER TOUGH® in Cleadent® (*P<.01). |
 | Fig. 2The pH curve of medium in which C. albicans grown on acrylic resin and resilient liners immersed in distilled water, Polident®, Cleadent®. No significant differences were observed among groups immersed in distilled water and denture cleansers (P>.05). |
 | Fig. 3Binding levels of radioisotope-labeled C. albicans on acrylic resin and resilient liners immersed in distilled water and denture cleanser. Except for GC RELINE™ and SOFRELINER TOUGH® immersed in Cleadent®, all the specimens immersed in denture cleansers exhibited significantly higher capacity of fungal biofilm formation than the control specimens (in distilled water) (*P<.01). |
Table 1
Acrylic resin and resilient liners used in this study

Table 2
Denture cleansers in this study

Table 3
Average surface roughness (Ra) of acrylic resin and resilient liners immersed in denture cleansers
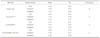
Table 4
Candida albicans binding level by radioisotope (ppm)

References
1. Budtz-Jörgensen E. Clinical aspects of Candida infection in denture wearers. J Am Dent Assoc. 1978; 96:474–479.
2. Bulad K, Taylor RL, Verran J, McCord JF. Colonization and penetration of denture soft lining materials by Candida albicans. Dent Mater. 2004; 20:167–175.
3. Nikawa H, Iwanaga H, Kameda M, Hamada T. In vitro evaluation of Candida albicans adherence to soft denture-lining materials. J Prosthet Dent. 1992; 68:804–808.
4. Nikawa H, Hamada T, Yamamoto T. Denture plaque-past and recent concerns. J Dent. 1998; 26:299–304.
5. Nikawa H, Hamada T, Yamashiro H, Kumagai H. A review of in vitro and in vivo methods to evaluate the efficacy of denture cleansers. Int J Prosthodont. 1999; 12:153–159.
6. Davenport JC, Wilson HJ, Spence D. The compatibility of soft lining materials and denture cleansers. Br Dent J. 1986; 161:13–17.
7. Harrison A, Basker RM, Smith IS. The compatibility of temporary soft materials with immersion denture cleansers. Int J Prosthodont. 1989; 2:254–258.
8. Goll G, Smith DE, Plein JB. The effect of denture cleansers on temporary soft liners. J Prosthet Dent. 1983; 50:466–472.
9. Nikawa H, Iwanaga H, Hamada T, Yuhta S. Effects of denture cleansers on direct soft denture lining materials. J Prosthet Dent. 1994; 72:657–662.
10. Verran J, Maryan CJ. Retention of Candida albicans on acrylic resin and silicone of different surface topography. J Prosthet Dent. 1997; 77:535–539.
11. Waters MG, Williams DW, Jagger RG, Lewis MA. Adherence of Candida albicans to experimental denture soft lining materials. J Prosthet Dent. 1997; 77:306–312.
12. Radford DR, Sweet SP, Challacombe SJ, Walter JD. Adherence of Candida albicans to denture-base materials with different surface finishes. J Dent. 1998; 26:577–583.
13. Nikawa H, Jin C, Makihira S, Egusa H, Hamada T, Kumagai H. Biofilm formation of Candida albicans on the surfaces of deteriorated soft denture lining materials caused by denture cleansers in vitro. J Oral Rehabil. 2003; 30:243–250.
14. Samaranayake LP. Nutritional factors and oral candidosis. J Oral Pathol. 1986; 15:61–65.
15. Nikawa H, Jin C, Hamada T, Murata H. Interactions between thermal cycled resilient denture lining materials, salivary and serum pellicles and Candida albicans in vitro. Part I. Effects on fungal growth. J Oral Rehabil. 2000; 27:41–51.
16. Nikawa H, Yamamoto T, Hayashi S, Nikawa Y, Hamada T. Growth and/or acid production of Candida albicans on soft lining materials in vitro. J Oral Rehabil. 1994; 21:585–594.
17. Nikawa H, Hamada T, Yamamoto T, Kumagai H. Effects of salivary or serum pellicles on the Candida albicans growth and biofilm formation on soft lining materials in vitro. J Oral Rehabil. 1997; 24:594–604.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download