Abstract
PURPOSE
The aim of this study was to evaluate the stability of arginine-glycine-aspartic acid (RGD) peptide coatings on implants by measuring the amount of peptide remaining after installation.
MATERIALS AND METHODS
Fluorescent isothiocyanate (FITC)-fixed RGD peptide was coated onto anodized titanium implants (width 4 mm, length 10 mm) using a physical adsorption method (P) or a chemical grafting method (C). Solid Rigid Polyurethane Foam (SRPF) was classified as either hard bone (H) or soft bone (S) according to its density. Two pieces of artificial bone were fixed in a customized jig, and coated implants were installed at the center of the boundary between two pieces of artificial bone. The test groups were classified as: P-H, P-S, C-H, or C-S. After each installation, implants were removed from the SRPF, and the residual amounts and rates of RGD peptide in implants were measured by fluorescence spectrometry. The Kruskal-Wallis test was used for the statistical analysis (α=0.05).
The stability of dental implants depends primarily on osseointegration at the bone-implant interphase.1 Since surface treatments of implants promote osseointegration and shorten the duration of osseointegration, various surface treatment techniques have been investigated.2-7 Cell adhesion plays an integral role in cell communication and regulation and is fundamentally important for the development and maintenance of cells. In particular, cell-material interactions influence the ability of cells that are in contact with materials to proliferate and differentiate.8 Interactions between an implant and surrounding cells are partly mediated by the production and deposition of bone specific extracellular matrix (ECM) proteins, such as, collagen type I and fibronectin.9 Interestingly, these ECM proteins are characterized by the arginine-glycine-aspartic acid (RGD) motif, which acts as a linkage between the ECM and anchorage-dependent osteoblastic cells, whose transmembrane integrin receptors attach to RGD motifs.10,11 As a consequence, transmembrane connections between the actin cytoskeleton and the RGD motif can activate several intracellular signaling pathways and modulate cell behavior, for example, by regulating proliferation, apoptosis, shape, mobility, gene expression, and differentiation.12 Due to this important role of the RGD sequence in cell adhesion, researchers have developed RGD peptide-immobilized Ti substrates to promote implant osseointegration.
Recently, two methods have been used to coat bioactive materials onto implant surfaces, that is, a passive coating method, which is based on covalent bond formation, and physical adsorption method that actively induces strong osseofixation and osseointegration at implant surfaces by immobilizing biochemical materials, such as, extracellular matrix (ECM) or growth factors on implant surfaces.2-4 Physical adsorption methods allow surfaces to be treated easily, but control of the amount and density of adsorbed bioactive material is problematic.13 Xiao et al.2 first introduced a method for chemically immobilizing silane on titanium surfaces. This chemical grafting method has the advantages of allowing concentrations of bioactive material on titanium surface to be predicted and the presentation/orientation of bioactive material and cells to be controlled.14,15 This method requires silane concentrations be optimized and the number of radicals on titanium surfaces to be increased. However, in order to chemically coat RGD onto anodized titanium surface, noxious chemicals like aminopropyltriethoxysilane (APTES), succinimidyl-4-[N-maleimidomethyl] cyclohexane-1-carboxylate (SMCC), and 4-(2-hydroxyethyl)-1-peperazine sulfuric acid (HEPES) are needed, and these may be toxic to humans.16
Bioactive materials on implant surfaces can be detached by physical stress at time of implant installation, and thus, bioactive materials must be physically stable on implant surfaces. Numerous studies have been conducted on the physical stabilities of inorganic coating on titanium.17,18 However, few studies have been conducted on the physical stability of bioactive materials on implant surfaces, and no study has been previously conducted on the stability bioactive material coatings on implant surfaces after installation.
Therefore, this study was conducted to assess the physical stabilities of RGD peptides coated onto anodized titanium surfaces using the chemical grafting and the physical adsorption method by measuring the amount of peptide remaining on titanium surfaces.
The peptide used in the experiment was synthesized by Peptron Inc. (Daejon, Korea) based on a specific sequence request. The peptide was composed of an RGD-based sequence of 14 bases-N-CAAALLLKERGDSK-C, and fluorescent isothiocyanate (FITC) was connected to its C-terminal as a marker. The cystein (C) group at the N-terminal was introduced for selective reactivity with the maleimide group used for chemical binding. The synthesized peptide was 86% pure by HPLC. It was protected from light in a lyophilized state by storing it in aluminum foil at -20℃ for < 3 months. The peptide was dissolved in distilled water before each experiment and used only once.
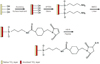
We used anodized Cell Nest® implants (Osstem, Pusan, Korea) that measured 4.0 mm in width and 10 mm in height. Implants coated with RGD peptides were minimally exposed to light to avoid hardening of the FITC, a fluorescence marker. Implants were divided into 2 groups for coating by physical adsorption or chemical grafting. In the physical adsorption group, the anodized implant surface was coated with RGD peptide by immersion in a solution containing 0.2 mg/mL of RGD peptide for 12 hours in the dark. Residual solution on coated implants were removed using wrinkle-free paper (KimWipes™, Kimberly-Clark Co., Irving, Texas), and implants were dried thoroughly in nitrogen atmosphere. In the chemical grafting group, implants were coated by chemical immobilization using Silane. After briefly activating anodized surfaces with UV/O cleaner (Jelight Co Inc, Irvine, CA, USA), implants were immersed for 90 minutes in a 2.5% (v/v) APTES ethanol solution. Implant surfaces were then rinsed with ethanol and dried in a nitrogen atmosphere at 110℃ for 1 hour. Implants were then reacted with 0.1 mg/mL Succinimidyl-4-[N-maleimidomethyl] cyclohexane-1-carboxylate (SMCC) for 1 hour in the dark, rinsed with PBS and distilled water, and dried in a nitrogen atmosphere. To ensure peptide adhesion, a buffer solution (pH 6.6) was prepared by adding 0.2 mg/mL 4-(2-hydroxyethyl)-1-piperazine sulfuric acid (HEPES) to 10 mM thiamine pyrophosphate (TPP). Because thiol radicals are oxidized to disulfide when they that react with the cystein radicals of RGD peptide and lose their reactivity to maleimide radicals, implants were reacted for more than 30 minutes with a HEPES buffer solution containing TPP, which reduces disulfide. Since amine and benzyl phenyl sulphide (BPS) inhibit the reaction between each other, neither was suitable for our experiment. Maleimide radical-inserted titanium treated by anodic oxidation was reacted with the aforementioned peptide solution. At the completion of the reaction, titanium was rinsed with HEPES buffer followed by distilled water and dried in a nitrogen atmosphere. A single layer of APTES was formed on the anodized titanium surface, and then coated with RGD peptide, which was immobilized on the implant surfaces by reacting the 2 radicals of SMCC with the amine radical of APTES and the thiol radical of the peptides, respectively (Fig. 1).
Anodized titanium discs (Osstem, Pusan, Korea) of diameter 10 mm and thickness 2 mm were used in this experiment. Roughnesses and surface areas of titanium discs were measured with a confocal laser scanning microscope (CLSM, OLS 3,000, Olympus, Tokyo) and a scanning microscope (FE-SEM, Stereoscan 440, Leica Cambridge, Cambridge, UK). Peptide coatings were confirmed by fluorescence microscopy (Nikon instruments Inc., Melville, NY, USA). Elemental constituents of the titanium discs were determined using an X-ray photospectrometer (XPS sigma probe ESCA, Thermo VG, West Sussex, UK). The brightness of each site was measured by fluorescence microscopy and coating homogeneity was confirmed. After 9 reference solutions were put in the cuvette, fluorescence intensity was measured using an excitation and emission wavelengths of 495 nm and 516 nm, respectively, using a fluorescence spectroscope (FP-6500, JASCO, Great Dunmow, UK). Standard concentration-fluorescence intensity curves were used to for quantification purposes.
Soft (S) and hard (H) artificial bones were used in this experiment. Solid rigid polyurethane foam (SRPF; Sawbones Worldwide, Pacific Research Laboratories, Inc., Vashon, Washington, USA) of density 0.32 g/cm3 was used as a soft cancellous bone (Grade 20, ASTM-F1839-08) and foam of density 0.64 g/cm3 was used as hard cortical bone (Grade 40, ASTM-F1839-08). In the hard bone group, hard cortical bone of SRPF was placed upper 1.5 mm to the soft cancellous bone (ASTM-F1839-08). The two pieces of artificial bone block were fixed using a customized jig, and coated implants were installed between the two pieces of artificial bone. Test groups were classified as: (1) physical adsorption and insertion in hard bone (P-H), (2) physical adsorption and insertion in soft bone (P-S), (3) chemical grafting and insertion in hard bone (C-H), and (4) chemical grafting and insertion in soft bone (C-S). Table 1 summarizes the experimental groups.
Twenty RGD peptide-coated implants were prepared per group, and 10 uncoated implants were prepared as controls. Implant installation was performed at the center of reattached site. An implantation machine (Osstem Implant, Pusan, Korea) was used to avoid surgical procedural differences. This machine was set at a given direction and rotation speed (30 rpm); drill burs up to 3.5 mm can be used for the installation of 4.0 mm implants (Fig. 2 and 3).
To exclude the interference by fluorescent material in the artificial bone itself, uncoated implants were installed and the amount of fluorescent material from artificial bone block on these implants was measured after removal (A). After installing RGD peptide-coated implants in the artificial bone block, amounts RGD peptide remaining and fluorescent material smeared from the artificial bone itself on removed implants were measured (B). Before RGD peptide-coated implants were installed, baseline RGD peptide amounts were measured (C). Amounts of RGD peptide lost after implant installation (Y) were calculated using the following formula:
Coating material remaining on implants was removed using 20% piperidine solution at room temperature for 30 minutes. For bone powder and RGD peptide-containing solutions, amounts of RGD peptides were measured in the presence of bone dust and after completing removing bone powder by centrifugation (13,000 rpm, 5 minutes).
Amounts of RGD peptide were measured by fluorescence spectroscopy. For quantitative analysis, each implant coated with RGD peptide was placed in a 1.5 mL Effendorf tube before and after installation. To elute RGD peptide from implant surfaces, 1 mL of 10% (v/v) ethanolamine was added (ethanolamine accelerates the detachment of RGD peptide by acting as a nucleophile at pH 11). Bone powder remaining on removed implants was detached by sonication for 5 minutes. After the bone dust was precipitated by centrifugation (6,400 rpm, 1 minute), 0.5 mL aliquots of supernatants were collected for quantitative analysis. Aliquot of supernatant were placed in 3 mL PL cuvettes (Sigma-Aldrich Inc., St Louis, MO, USA), and 2.0 mL of distilled water was added to a final volume of 2.5 mL. Absorbance was measured at excitation and emission wavelengths of 4.95 and 516 nm, respectively, using a fluorescence spectroscope (FP-65000, JASCO). To convert measured absorbances to peptide concentrations, standard reference solutions were used (Fig. 4).
Statistical analysis was performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was performed to test the normality of distribution, and the Kruskal-Wallis test was used for the statistical analysis (α=0.05). Bonferroni's post-hoc test was performed at a significance level of 95%.
Fig. 5 shows FE-SEM images of an anodized titanium surface after coating with RGD peptide. The anodized titanium surface was porous and smooth with a roughness of 0.26 ± 0.03 µm. However, surface structures (as determined by SEM) were similar in the experimental groups. Fig. 6 shows fluorescent microscopic images after RGD peptide coating. No fluorescent material was detected on the surfaces of uncoated titanium implants. Fluorescent material was evident on all RGD peptide coated implant surfaces, with statistical significance. Implant surfaces in the physical adsorption group fluoresced more than in the chemical grafting group. Implant surfaces were yellower in the physical coated groups. Green dot-like areas were sparsely present in both the physical adsorption and chemical grafting groups.
Atomic elemental analysis of substrates was performed by XPS. Results are expressed percentage atomic compositions (Table 2). Elemental silicon was detected only on APTES and chemically coated substrates. Elemental nitrogen was increased by both physical adsorption and chemical grafting methods. Elemental carbon and nitrogen were more increased by physical coating at high peptide levels.
Before implant installation, the amount of RGD peptide on the implant surfaces was higher in the P-H and P-S groups than in the C-H and C-S groups (518.4 ± 40.8 ng versus 224.0 ± 25.12 ng, respectively; P<.05). Table 3 shows the residual amounts of RGD peptide in the experimental groups. Values were highest in the P-S group, followed by the P-H, C-S and C-H groups.
The residual ratios of RGD peptide were significantly different between the experimental groups. There were significant differences in the residual rates of RGD peptide between the P-S group and the other groups (63.19% ± 12.20% in the P-S group, 32.54 ± 2.45% in the C-S group, 37.27% ± 7.05% in the P-H group, and 30.00% ± 4.57% in the C-H group) (P<.05).
Integrin, a cell membrane receptor, is involved in adhesion between cells and extracellular matrix proteins.19 Integrin interacts by using a short sequence of amino acids within the extracellular matrix. In particular, the sequence arg-glyasp (RGD) mediates adhesion between cells and plasma or extracellular matrix proteins (fibronectin, vitronectin, type I collagen, osteopontin, and bone sialoprotein).20 Synthetic peptides containing RGD also have similar activities.21,22 The recognition of cells by small and simple peptides is useful for increasing cell-implant interactions by enabling the surface of implants to adhere to surrounding cells. Furthermore, it has been shown that interactions between bioactive materials and cells can be promoted by coating implant surfaces with peptides.23
Proteins attached to implant surfaces can be lost during or after implant installation. To overcome this problem, methods involving the covalent attachment of bioactive materials to substrates containing radicals have been developed. In this study, to fabricate RGD peptide coated-anodized Ti, the immobilization of RGD peptide onto anodized Ti was performed by physical adsorption and chemically. Simply dipping and drying was used to coat RGD onto anodized Ti surfaces by physical adsorption due to van der Waals, hydrophobic, or electrostatic forces.24 In order to chemically coat RGD onto anodized Ti surfaces, Ti surfaces are activated with APTES and then reacted with SMCC crosslinker before RGD peptide binding.16 RGD peptide is chemically immobilized on anodized Ti surface by reaction between its thiol group and the maleimide group of surface bound SMCC. As shown in Table 2, the C and N contents of RGD coated Ti substrates were greater than those of anodized Ti. The main differences between chemical grafting and physical adsorption are the silicon content and C-N-H bond formation due to APTES deposition. XPS results confirmed that RGD peptide was successfully immobilized onto anodized Ti surfaces by both methods. In particular, fluorescence microscopy showed that overall brightness were higher in the physical adsorption group. The green dots observed at long wavelength were probably due to the presence of aggregated peptides and the slightly dark areas were probably due to surface damage during surface treatment. In the chemical grafting group, fluorescence emission was lower, and color was green closer to a short wavelength. In addition, few dark areas were sparsely seen, similar to the physical adsorption group, and the proportion of dots was low, presumably due to the washing process. In summary, amounts of RGD peptide on implant surfaces were higher in the physical adsorption group than in the chemical grafting group.
Measurements of adhesive strength are useful for evaluating implant surfaces coated with bioactive materials. Current standard methods for assessing the adhesive strengths of bioactive materials, include the tape test (ASTM D3359, Standard Test Method for Measuring Adhesion), and shear strength measurement (ASTM F1044, Test Method for Shear Testing of Calcium Phosphate and Metallic Coatings). These methods are used to evaluate inorganic substances because the tapes and epoxy resin are strong chemical adhesives. Thus, if these substances are used to evaluate implant surfaces coated with organic bioactive materials, they could affect chemical bonding and cause denaturation of bioactive materials, and thus, adversely affect results. Implant surfaces treated using the aforementioned inorganic substances exhibited irregular ultra-fine structures. In addition, it is unclear whether inorganic substances can penetrate deep into implant surfaces. However, there is still no standard method for evaluating implant surfaces coated with organic bioactive materials. For this reason, we sought to establish a technique for measuring the amount of bioactive materials coated on implant surfaces and the amounts that are shed from surfaces. We found that residual amounts of RGD peptide on implant surfaces were larger in the physical adsorption group and that the residual rate of peptide after implant installation was also higher in this group. Further studies on the physical strengths of peptide coatings attached to implant surface by covalent bond are warranted.
The results of this study indicate that the amount of RGD peptide coated on anodized titanium surfaces depends on the coating method used. In particular, the physical adsorption method was found to attach larger amounts of RGD peptide to surfaces to provide more stable coatings than chemical grafting.
Figures and Tables
 | Fig. 2Implant installation using a machine developed by Osstem Co. (Pusan, Korea). This machine was set at a given direction and rotation speed (30 rpm); drill burs up to 3.5 mm can be used for the installation of 4.0 mm implants. |
 | Fig. 3Divided surface of a SRPF; A: After implant installation, B: each SRPF was divided into 2 pieces and the implant was removed. |
 | Fig. 4Schematic diagram of RGD-peptide quantitation.
B - A = amount of RGD peptide remaining on an implant after installation.
C - (B - A) = amount of RGD peptide lost from the implant surface.
|
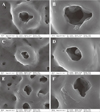 | Fig. 5SEM micrographs of each group, no differences were observed after coating with RGD peptide; A&B: bare anodized titanium, C&D: physical adsorption group, E&F: chemical grafting group; A,C,E: ×50,000, B,D,F: ×100,000. |
 | Fig. 6Fluorescence microscopic images after RGD-peptide coating (A: bare anodized titanium, B: physical adsorption group, C: chemical grafting group, bar: 200 µm). Fluorescence was greater in the physical adsorption group than in the chemical grafting group. Implants surfaces were yellower in the physical adsorption group. Green dot-like areas were sparsely present in both the physical adsorption and chemical grafting groups. |
References
1. Albrektsson T, Brunski J, Wennerberg A. 'A requiem for the periodontal ligament' revisited. Int J Prosthodont. 2009; 22:120–122.
2. Xiao SJ, Textor M, Spencer ND, Wieland M, Keller B, Sigrist H. Immobilization of the cell-adhesive peptide Arg-Gly-Asp-Cys (RGDC) on titanium surfaces by covalent chemical attachment. J Mater Sci Mater Med. 1997; 8:867–872.
3. Rezania A, Healy KE. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J Biomed Mater Res. 2000; 52:595–600.
4. Bab I, Chorev M. Osteogenic growth peptide: from concept to drug design. Biopolymers. 2002; 66:33–48.
5. Tsuchimoto Y, Yoshida Y, Takeuchi M, Mine A, Yatani H, Tagawa Y, Van Meerbeek B, Suzuki K, Kuboki T. Effect of surface pre-treatment on durability of resin-based cements bonded to titanium. Dent Mater. 2006; 22:545–552.
6. Jang HW, Kang JK, Lee K, Lee YS, Park PK. A retrospective study on related factors affecting the survival rate of dental implants. J Adv Prosthodont. 2011; 3:204–215.
7. Pak HS, Yeo IS, Yang JH. A histomorphometric study of dental implants with different surface characteristics. J Adv Prosthodont. 2010; 2:142–147.
8. Nguyen MN, Lebarbe T, Zouani OF, Pichavant L, Durrieu MC, Héroguez V. Impact of RGD nanopatterns grafted onto titanium on osteoblastic cell adhesion. Biomacromolecules. 2012; 13:896–904.
9. Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007; 28:3228–3235.
10. Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987; 48:549–554.
11. Grigoriou V, Shapiro IM, Cavalcanti-Adam EA, Composto RJ, Ducheyne P, Adams CS. Apoptosis and survival of osteoblast-like cells are regulated by surface attachment. J Biol Chem. 2005; 280:1733–1739.
12. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Integrins: bidirectional, allosteric signaling machines. Cell. 2002; 110:673–687.
13. Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007; 28:3228–3235.
14. Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003; 24:4385–4415.
15. Bagno A, Piovan A, Dettin M, Chiarion A, Brun P, Gambaretto R, Fontana G, Di Bello C, Palù G, Castagliuolo I. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone. 2007; 40:693–699.
16. Porté-Durrieu MC, Guillemot F, Pallu S, Labrugère C, Brouillaud B, Bareille R, Amédée J, Barthe N, Dard M, Baquey Ch. Cyclo-(DfKRG) peptide grafting onto Ti-6Al-4V: physical characterization and interest towards human osteoprogenitor cells adhesion. Biomaterials. 2004; 25:4837–4846.
17. Mustafa K, Wennerberg A, Wroblewski J, Hultenby K, Lopez BS, Arvidson K. Determining optimal surface roughness of TiO(2) blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin Oral Implants Res. 2001; 12:515–525.
18. Sul YT, Johansson C, Wennerberg A, Cho LR, Chang BS, Albrektsson T. Optimum surface properties of oxidized implants for reinforcement of osseointegration: surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int J Oral Maxillofac Implants. 2005; 20:349–359.
19. LeBaron RG, Athanasiou KA. Extracellular matrix cell adhesion peptides: functional applications in orthopedic materials. Tissue Eng. 2000; 6:85–103.
20. Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994; 9:487–496.
21. Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984; 309:30–33.
22. Pierschbacher MD, Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci USA. 1984; 81:5985–5988.
23. Massia SP, Hubbell JA. Covalently attached GRGD on polymer surfaces promotes biospecific adhesion of mammalian cells. Ann N Y Acad Sci. 1990; 589:261–270.
24. Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003; 24:4385–4415.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download