1. Wataha JC. Biocompatibility of dental casting alloys: a review. J Prosthet Dent. 2000; 83:223–234. PMID:
10668036.

2. Pretti M, Hilgert E, Bottino MA, Avelar RP. Evaluation of the shear bond strength of the union between two CoCr-alloys and a dental ceramic. J Appl Oral Sci. 2004; 12:280–284. PMID:
20976397.

3. Grimaudo NJ. Biocompatibility of nickel and cobalt dental alloys. Gen Dent. 2001; 49:498–503. PMID:
12017794.
4. Dobrzański LA, Reimann L. Influence of Cr and Co on hardness and corrosion resistance CoCrMo alloys used on dentures. J Achieve Mater Manuf Eng. 2011; 49:193–199.
5. Karpuschewski B, Pieper HJ, Krause M, Döring J. In : Schuh G, Neugebauer R, Uhlmann E, editors. Future trends in production engineering. 1st ed. Proceedings of the first conference of the German Academic Society for production engineering; 8th-9th June 2011; Berlin, Germany. New York: Springer;2013.
6. Fasbinder D. Using digital technology to enhance restorative dentistry. Compend Contin Educ Dent. 2012; 33:666–668. PMID:
23030729.
7. Miyazaki T, Hotta Y, Kunii J, Kuriyama S, Tamaki Y. A review of dental CAD/CAM: current status and future perspectives from 20 years of experience. Dent Mater J. 2009; 28:44–56. PMID:
19280967.

8. Aboushelib MN, Elmahy WA, Ghazy MH. Internal adaptation, marginal accuracy and microleakage of a pressable versus a machinable ceramic laminate veneers. J Dent. 2012; 40:670–677. PMID:
22542500.

9. Figliuzzi M, Mangano F, Mangano C. A novel root analogue dental implant using CT scan and CAD/CAM: selective laser melting technology. Int J Oral Maxillofac Surg. 2012; 41:858–862. PMID:
22377004.

10. Jevremovic D, Puskar T, Kosec B, Vukelic D, Budak I, Aleksandrovic S, Egbeer D, Williams R. The analysis of the mechanical properties of F75 Co-Cr alloy for use in selective laser melting (SLM) manufacturing of removable partial dentures (RPD). Metal. 2012; 51:171–174.
11. Hollander DA, von Walter M, Wirtz T, Sellei R, Schmidt-Rohlfing B, Paar O, Erli HJ. Structural, mechanical and in vitro characterization of individually structured Ti-6Al-4V produced by direct laser forming. Biomaterials. 2006; 27:955–963. PMID:
16115681.

12. Rodrigues WC, Broilo LR, Schaeffer L, Knörnschild G, Espinoza FRM. Powder metallurgical processing of Co-28%Cr-6%Mo for dental implants: Physical, mechanical and electrochemical properties. Powder Tech. 2011; 206:233–238.

13. Bauer JR, Grande RH, Rodrigues-Filho LE, Pinto MM, Loguercio AD. Does the casting mode influence microstructure, fracture and properties of different metal ceramic alloys? Braz Oral Res. 2012; 26:190–196. PMID:
22641437.

14. Gill P, Munroe N, Pulletikurthi C, Pandya S, Haider W. Effect of Manufacturing Process on the Biocompatibility and Mechanical Properties of Ti-30Ta Alloy. J Mater Eng Perform. 2011; 20:819–823. PMID:
21666859.

15. Manfredi D, Calignano F, Krishnan M, Canali R, Ambrosio EP, Atzeni E. From powders to dense metal parts: Characterization of a commercial AlSiMg alloy processed through direct metal laser sintering. Materials. 2013; 6:856–869.

16. Tandon R. Disegi JA, Kennedy RL, Pilliar R, editors. Cobalt Base Alloys for Biomedical Applications ASTM STP 1365. West Conshohocken, PA: American Society for Testing and Materials;1999.
17. Bolzoni L, Esteban PG, Ruiz-Navas EM, Gordo E. Mechanical behaviour of pressed and sintered titanium alloys obtained from master alloy addition powders. J Mech Behav Biomed Mater. 2012; 15:33–45. PMID:
23026730.

18. Lohfeld S, McHugh PE. Laser sintering for the fabrication of tissue engineering scaffolds. Methods Mol Biol. 2012; 868:303–310. PMID:
22692618.

19. Castillo-de-Oyagüe R, Sánchez-Turrión A, López-Lozano JF, Albaladejo A, Torres-Lagares D, Montero J, Sáarez-García MJ. Vertical misfit of laser-sintered and vacuum-cast implant-supported crowncopings luted with definitive and temporary luting agents. Med Oral Patol Oral Cir Bucal. 2012; 17:e610–e617. PMID:
22322524.
20. Traini T, Mangano C, Sammons RL, Mangano F, Macchi A, Piattelli A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent Mater. 2008; 24:1525–1533. PMID:
18502498.

21. Girardin E, Renghini C, Dyson J, Calbucci V, Moroncini F, Albertini G. Characterization of porosity in a laser sintered MMCp using X-ray synchrotron phase contrast microtomography. Mater Sci Appl. 2011; 2:1322–1330.

22. Gaytan SM, Murr LE, Martinez E, Martinez JL, Machado BI, Ramirez DA, Medina F, Collins S, Wicker RB. Comparison of Microstructures and mechanical properties for solid and mesh cobalt-base alloy prototypes fabricated by electron beam melting. Metall Mater Trans A. 2010; 41:3216–3227.

23. Chen CL, Tatlock GJ, Jones AR. Effect of annealing temperatures on the secondary re-crystallization of extruded PM2000 steel bar. J Microsc. 2009; 233:474–481. PMID:
19250468.

24. Guo WH, Brantley WA, Li D, Clark WA, Monaghan P, Heshmati RH. Annealing study of palladium-silver dental alloys: Vickers hardness measurements and SEM microstructural observations. J Mater Sci Mater Med. 2007; 18:111–118. PMID:
17200820.

25. Porod G. Glatter O, Kratky O, editors. Small angel X-ray scattering. London, UK: Academic Press Inc;1982.
26. Martin JE, Hurd AJ. Scattering from fractals. J Appl Crystallogr. 1987; 20:61–78.

27. EN ISO 18265: 2005. Metallic materials - Conversion of hardness values.
28. Bezzon OL, Ribeiro RF, Rollo JM, Crosara S. Castability and resistance of ceramometal bonding in Ni-Cr and Ni-Cr-Be alloys. J Prosthet Dent. 2001; 85:299–304. PMID:
11264939.

29. Kern M, Thompson VP. Durability of resin bonds to a cobalt-chromium alloy. J Dent. 1995; 23:47–54. PMID:
7876416.

30. Wataha JC, Lockwood PE. Release of elements from dental casting alloys into cell-culture medium over 10 months. Dent Mater. 1998; 14:158–163. PMID:
10023206.

31. Wataha JC, Nelson SK, Lockwood PE. Elemental release from dental casting alloys into biological media with and without protein. Dent Mater. 2001; 17:409–414. PMID:
11445208.

32. Liu R, Xi SQ, Kapoor S, Wu XJ. Effects of chemical composition on solidification, microstructure and hardness of Co-Cr-W-Ni and Co-Cr-Mo-Ni alloy systems. Int J Res Rev Appl Sci. 2010; 5:110–122.
33. Iseri U, Ozkurt Z, Kazazoglu E. Shear bond strengths of veneering porcelain to cast, machined and laser-sintered titanium. Dent Mater J. 2011; 30:274–280. PMID:
21597225.

34. Heat Treating. ASM Handbook. Vol. 4. Ohio: ASM International;1991.
35. Tani T, Udoh K, Yasuda K, Van Tendeloo G, Van Landuyt J. Age-hardening mechanisms in a commercial dental gold alloy containing platinum and palladium. J Dent Res. 1991; 70:1350–1357. PMID:
1939828.

36. Kim HI, Lee DH, Sim JS, Kwon YH, Seol HJ. Age-hardening by miscibility limit of Au-Pt and Ag-Cu systems in an Au-Ag-Cu-Pt alloy. Mater Character. 2009; 60:357–362.

37. Nomura N, Abe M, Kawamura A, Fujinuma S, Chiba A, Masahashi N, Hanada S. Fabrication and mechanical properties of porous Co-Cr-Mo alloy compacts without Ni addition. Mater Trans. 2006; 47:283–286.
38. Telu S, Patra A, Sankaranarayana M, Mitra R, Pabi SK. Microstructure and cyclic oxidation behavior of W-Cr alloys prepared by sintering of mechanically alloyed nanocrystalline powders. Int J Refract Met Hard Mater. 2013; 36:191–203.

39. Mackert JR Jr, Ringle RD, Fairhurst CW. High-temperature behavior of a Pd-Ag alloy for porcelain. J Dent Res. 1983; 62:1229–1235. PMID:
6581200.

40. McGinley EL, Coleman DC, Moran GP, Fleming GJ. Effects of surface finishing conditions on the biocompatibility of a nickel-chromium dental casting alloy. Dent Mater. 2011; 27:637–650. PMID:
21514653.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


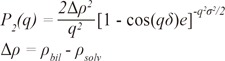

 XML Download
XML Download