Abstract
PURPOSE
The aim of this study was to evaluate the effects of repeated porcelain firing process on the corrosion rates of the dental alloys.
MATERIALS AND METHODS
Cr-Co, Cr-Ni and Pd-Ag alloys were used for this study. Each metal supported porcelain consisted of 30 specimens of 10 for 7, 9 and 11 firing each. Disc-shaped specimens 10 mm diameter and 3 mm thickness were formed by melting alloys with a propane-oxygen flame and casted with a centrifuge casting machine and then with the porcelain veneer fired onto the metal alloys. Corrosion tests were performed in quintuplicate for each alloy (after repeated porcelain firing) in Fusayama artificial saliva solution (pH = 5) in a low thermal-expansion borosilicate glass cell. Tamhane and Sheffe test was used to compare corrosion differences in the results after repeated firings and among 7, 9 and 11 firing for each alloy. The probability level for statistical significance was set at α=0.05.
Many different types of alloys are available in the dental market, which can be used for prosthetic applications. The most important factors affecting the choice of these alloys are mechanical properties, workability, biocompatibility and resistance to corrosion.1 Some metallic elements are completely safe in the elemental state, but on the other hand, they can form harmful, toxic ions or compounds to the body. Therefore, in developed countries noble metals and all ceramic materials are mostly used to keep away from detrimental effects of non precious metals. Because noble metals possess good resistance to corrosion due to low reactivity and compatible biological properties, in developing countries non-precious alloys such as Cobalt Chromium (Co-Cr), Chromium Nickel (Cr-Ni) have been preferred because of their cheap costs.2 Cobalt-based alloys provide strength, hardness and resistance to corrosion. Chromium provides corrosion resistance when its concentration is between 16 and 20 wt%.3
Nickel increased modulus elasticity of the casting alloys and also thermal expansion coefficient of Nickel based alloys and it is consistent for conventional porcelains, which prevents cracking of the veneer during firing.4 Nickel is allergenic material so Nickel sensitivity is thought to be potential clinical effect for these alloys. For this reason, Nickel ion released during corrosion is much more important than other metals for this reason.
Porcelain veneer fired onto the metal alloys for aesthetic purposes referred to as porcelain-fused to metal (PFM). Sometimes only facial aspect of the metal veneered while the occlusal and lingual aspects are left intact. Alternatively, only the lingual aspect close proximity to the gingiva are left as half moon shape for gingival accordance since metal release through corrosion process may cause adverse reactions.5,6
Heat treatments for metal alloys and PFM firing process effects alloy surface oxides, microstructures and physical properties.7-9 Therefore it is important to evaluate the effects of porcelain firing process on the corrosion of dental metal alloys.
There are some studies about the porcelain firing process on the corrosion and surface properties of the dental alloys8,10 and some studies carried out about their corrosion behavior in artificial saliva.3,11 On the other hand, there is no study about the effect of the number of firing on dental alloys' corrosion resistance. The purpose of this study was to evaluate the effects of repeated porcelain firing process on the corrosion rates of the dental alloys. The research hypothesis was that corrosion resistance would occur relative to the firing times and among alloys.
Cr-Co (Wirobond C; Bego Dental, Bremen, Germany), Cr-Ni (Shera; GMBH&Co. KG, Germany) and Pd-Ag (Begopal 300; Bego, GMBH&Co. KG, Bremen, Germany) alloys were used for this study. Each metal supported porcelain consisted of 30 specimens of 10 for 7, 9 and 11 times firing each. Totally 90 specimens fabricated for this study. The compositions of these alloys were shown in Table 1. All three alloys can be used for fabricating metal-porcelain restorations. The castings were prepared in accordance with the manufacturers' recommendations in the dental laboratory. 10 mm diameter and 3 mm thickness disc-shaped specimens were formed by melting alloys with a propane-oxygen flame and cast with a centrifuge casting machine (Motorcast, Degussa, Germany). The cast specimens were wet-polished using silicon carbide abrasive sandpaper up to 1500 grit to simulate clinical procedures,6 then ultrasonically cleaned for 5 minutes each in acetone, ethanol, and de-ionized water to eliminate surface contaminants.
Porcelain veneer was applied on the metal alloys, then heat was applied on the specimens under vacuum in a dental porcelain furnace (Ivoclar Vivadent P-300 Leciester, UK). The specimens were degassed at 1,010℃ under vacuum for 5 minutes, opaque fired at 980℃ under vacuum and air cooled, body fired at 970℃ under vacuum and air cooled, a moon shape metal was leaved on the specimens to simulate porcelain crowns in the mouth and finally glaze fired at 980℃ and air cooled.9 Moon shape metal parts were polished with rubbers. Specimens were fired 7, 9 and 11 times respectively and corrosion resistance was recorded. SEM evaluations were acquired before and after number of firing process.
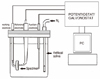
Corrosion measurements were performed using an electrochemical potentiostat/galvanostat (PARSTAT 2273; Princeton Applied Research, Oak Ridge, TN, USA) via a test cell with the mounted specimen as the working electrode, a high-purity platinum wire as the counter electrode, and Saturated Calomel Electrode (SCE) as the reference electrode (Fig. 1). Corrosion tests were performed in quintuplicate for each alloy (after porcelain firing) in Fusayama artificial saliva solution (0.4 g l-1 NaCl, 0.4 g l-1 KCl, 0.795 g l-1 CaCl2·2H2O, 0.690 g l-1 NaH2PO4·H2O, 0.005 g l-1 Na2S·9H2O, 1.0 g l-1 urea, pH 5.0) in a Pyrex glass cell. Samples were polished with successively finer grade of emery papers (up to 800 grit) and then degreased with toluene. The experiments were carried out at ambient temperature. The corrosion behaviors of the samples were investigated by both Tafel and open circuit potential (OCP) methods. OCPs were measured in the electrolytes before carrying out the experiments. The OCP was measured for duration of 3,000 seconds. The exposed areas of the specimens were about 0.1 cm2. SCE with scan rate of 0.166 mV/sec. The breakdown potential (Ebr), at which the corrosion current increases abruptly, was also estimated from the polarization curves. Icorr and Rp represent the corrosion rate and corrosion resistance, respectively (Table 2). Because the breakdown of passive oxide film, marked by a large and generally increasing current, is caused by localized or pitting-type corrosion, the corrosion resistance of the alloys can also be evaluated using the breakdown potential, which means that a greater Ebr value indicates a better corrosion resistance.12
Statistical analysis data from three different metal alloys for corrosion testing were analyzed statistically with SPSS v11.5 software (SPSS, Chicago, IL, USA). Tamhane and Sheffe test was used to compare corrosion differences in results after repeated firings and among 7, 9 and 11 firing for each alloys. The probability level for statistical significance was set at α = 0.05.
Fig. 2A shows the time potential plots of Cr-Co dental materials viz. (i) 7, (ii) 9, and (iii) 11, exposed to artificial saliva solution. Open circuit potentials are one measure used to determine the potential at which the anodic and cathodic corrosion reactions cancel one another out. These values indicate a metal's tendency to corrode in the given electrolyte. Upon exposure of these samples in artificial saliva, it was observed that all materials except 11 times firing showed a shift in the potential toward noble direction. The Ecorr shift was maximum (30 mV) in case of 7 times firing (Commercial) while minimum Ecorr shift was observed in case of 11 times firing (5 mV). This is possibly due to better passive film formation in 7 times firing. Finally, the order of Ecorr after stabilization was 7 > 9 > 11 (P<.05).
The high temperatures reached during porcelain firing may cause change in the form or structure of the composition of the surface oxides, and alter the corrosion properties of the alloy.
Fig. 2B shows the corrosion property of electrodeposited Cr-Co series alloy in a fusayama solution. The corrosion potential (Ecorr) of the samples are -0.33 VSCE for the 11 times firing and -0.24 VSCE 7 times firing. Compared with 11 firing sample, it is found that the corrosion potential of the deposited at 7 firing sample is 37% nobler. It is thus further concluded that the firing times of the Cr-Co series alloys up to firing time 7 possesses superior anti-corrosion behaviors than that of firing times 11 (P<.05). Corrosion current was maximum in 11 times fired alloy. It can be seen from the Fig. that corrosion rate of fired Cr-Co was at minimum possibly because of better spontaneous passive film formation due to chromium oxide formation on the film surface or possible phase transformation of cast alloy.
Fig. 2C shows the corrosion property of Cr-Ni series alloy in a fusayama solution. The corrosion potential (Ecorr) of the smallest corrosive resistive at 11 firing and the biggest corrosion resistive 7 firing are -0.48 VSCE and -0.13 VSCE, respectively (P<.05). Compared with 11 firing sample, it is found that the corrosion potential of the deposited at 7 firing sample is 269% nobler. It is thus further concluded that the firing times of the Cr-Ni series alloys up to firing time 7 possesses superior anti-corrosion behaviors than 11 times firing.
Fig. 2D shows the corrosion property of electrodeposited Pd-Au series alloy in a fusayama solution. The corrosion potential (Ecorr) of the smallest corrosive resistive at 11 firing and the biggest corrosion resistive Pd-Ag 7 firings are -0.35 VSCE and 0.14 VSCE, respectively (P<.05). Compared with 11 firing sample, it was found that the corrosion potential of the deposited at 7 firing sample is 350% nobler. It is thus further concluded that the firing times of the Pd-Ag series alloys up to firing time 7 possesses superior anti-corrosion behaviors than that of firing times 11. It was evident from the Fig. that the Tafel behavior of all these alloys is not highly distinct.
Figs. 3, 4 and 5 show the Scanning Electron Microscopy images (SEM) of Cr-Co, Pd-Ag and Cr-Ni series before and after corrosion tests, respectively. In the SEM images, alloys show obvious changes on their surface morphology after polarized in the solution. Especially at 11 times fired sample has an protective oxide layer.
This in vitro study measured the corrosion resistance of the alloys according to firing numbers. The results of this study supports the hypothesis that corrosion resistance would change relative to the firing numbers and the corrosion resistance of the alloy were affected by the firing numbers differently.
Corrosion of dental alloys are affected by multi-factorial conditions such as alloy's composition, recurrent castings,1 environmental conditions, and composition of the surrounding electrolyte selected for study5,13 so the same pH (5) and electrolyte medium were used for this study.
Noble alloys are defined as having a noble metal (gold, platinum, palladium) content greater than or equal to 25% by weight14 and have more resistance to corrosion than base metal alloys because of low reactivity, resulting from the noble nature of the atoms. This idea was supported by Manaranche and Hornberger's study15 but these are very expensive and cannot be used routinely in dentistry. Base metals, such as molybdenum, chromium, and nickel, have a great affinity for oxygen and thus form a thin passivating oxide film that acts as a protective layer from corrosion.16 Therefore they can be used for fixed prosthesis instead of noble alloys.
As a result of this study, Cr-Ni alloy resulted in lower corrosive rate than Cr-Co alloy. Cr amounts are the same and this can be explained by its higher percentage of Mo of Cr-Ni alloy. Mo as molybdenum oxide (Mo2O3) and as Cr as chromium oxide (Cr2O3) provide the initial stability to prevent dissolution of metal ions and thus provide resistance to corrosion and lesser corrosive rate.17
Molybdenum, chromium and nickel alloys can be added to promote resistance to corrosion; in contrast, small variations in their compositions affect this corrosion resistance.1 In dental alloys, the ratio of Cr and Mo ranges from 11 to 25 and 0 to 10 wt% respectively. Under this ratio it was reported that alloys were more susceptible to corrosion.18
Porcelain firing to metal process affects the alloy's resistance to corrosion. This may be due to increased levels of the released metal ions from the samples. According to studies by Wylie et al.19 and Lin HY et al.,10 despite the small changes in the alloy microstructures and increased ion release after porcelain firing, no differences in corrosion behaviour were detected. On the other hand, Roach et al.8 reported that porcelain firing had a detrimental effect on the corrosion properties of alloys, which is in consistence with this study.
Surface analysis of the alloys was not done in this study; only the corrosion resistance were measured. Unlike other studies, porcelain was fired at an increasing and different numbers (7, 9 and 11) in this study and proportionally ones increased porcelain firing number, alloy's corrosion resistance was decreased. This may be because the fixed prosthesis requires high temperature firing cycles which results in changes in the surface structure during the porcelain firing processes8,20,21 and when firing process is repeated, a negative effect of high temperature increased on the alloys.
The limitation of this study was that fusayama artificial saliva solution provides only the inorganic components, and does not consist of organic components; however, this electrolyte has a response close to natural saliva.22 The actual conditions of the oral environment is very complex. Therefore, it was difficult to simulate the same composition of oral environment.23
Thus alloys such as Cr-Ni can be corrosive with regard to in vitro study.19 On the other hand, it can be reported that there is good corrosion resistance for Cr-Ni casting alloys in the oral cavity.24 Porcelain firing should be considered important when evaluating the corrosion behavior of dental alloys. Therefore, further in vivo studies about corrosion may be required.
The nature of dental alloys plays a major role in the corrosion resistance rate. On comparing corrosion resistance of the materials after repeated firings, the results were Pd-Ag > Cr-Ni > Cr-Co respectively. Repeated firings decreased corrosion resistance of Pd-Ag, Cr-Co and Cr-Ni alloys. Therefore, dentists should keep away from repeated firings if possible while fabricating the fixed prosthesis.
Figures and Tables
 | Fig. 2A: Time potential curves of Cr-Co alloys, B: Potentiodynamic polarisation of cast Cr-Co series in the fusayama test solutions, C: Potentiodynamic polarisation of cast Cr-Ni series in the fusayama test solutions, D: Current density-potential curves (Tafel plots) of various firing counts for Pd-Ag series. |
 | Fig. 3SEM images of the Cr-Co dental alloys before and after corrosion test (Original magnification ×1,000). A: before the corrosion test, B: after the corrosion test. |
 | Fig. 4SEM images of the Pd-Ag dental alloys before and after corrosion test (Original magnification ×1,000). A: before the corrosion test, B: after the corrosion test. |
References
1. Kedici SP, Aksüt AA, Kílíçarslan MA, Bayramoğlu G, Gökdemir K. Corrosion behaviour of dental metals and alloys in different media. J Oral Rehabil. 1998. 25:800–808.
2. Elshahawy W, Watanabe I, Koike M. Elemental ion release from four different fixed prosthodontic materials. Dent Mater. 2009. 25:976–981.
3. Viennot S, Dalard F, Lissac M, Grosgogeat B. Corrosion resistance of cobalt-chromium and palladium-silver alloys used in fixed prosthetic restorations. Eur J Oral Sci. 2005. 113:90–95.
4. McLean J. The science and art of dental ceramics. The nature of dental ceramics and their clinical use. 1979. 1st ed. Chicago: Quintessence Publishing.
5. Schmalz G, Garhammer P. Biological interactions of dental cast alloys with oral tissues. Dent Mater. 2002. 18:396–406.
6. Wataha JC. Biocompatibility of dental casting alloys: a review. J Prosthet Dent. 2000. 83:223–234.
7. Johnson T, van Noort R, Stokes CW. Surface analysis of porcelain fused to metal systems. Dent Mater. 2006. 22:330–337.
8. Roach MD, Wolan JT, Parsell DE, Bumgardner JD. Use of x-ray photoelectron spectroscopy and cyclic polarization to evaluate the corrosion behavior of six nickel-chromium alloys before and after porcelain-fused-to-metal firing. J Prosthet Dent. 2000. 84:623–634.
9. Bumgardner JD, Lucas LC. Surface analysis of nickel-chromium dental alloys. Dent Mater. 1993. 9:252–259.
10. Lin HY, Bowers B, Wolan JT, Cai Z, Bumgardner JD. Metallurgical, surface, and corrosion analysis of Ni-Cr dental casting alloys before and after porcelain firing. Dent Mater. 2008. 24:378–385.
11. Reclaru L, Lüthy H, Eschler PY, Blatter A, Susz C. Corrosion behaviour of cobalt-chromium dental alloys doped with precious metals. Biomaterials. 2005. 26:4358–4365.
12. Matković T, Matković P, Malina J. Effects of Ni and Mo on the microstructure and some other properties of Co-Cr dental alloys. J Alloys Compd. 2004. 366:293–297.
13. Huang HH. Effect of chemical composition on the corrosion behavior of Ni-Cr-Mo dental casting alloys. J Biomed Mater Res. 2002. 60:458–465.
14. Beck KA, Sarantopoulos DM, Kawashima I, Berzins DW. Elemental release from CoCr and NiCr alloys containing palladium. J Prosthodont. 2012. 21:88–93.
15. Manaranche C, Hornberger H. A proposal for the classification of dental alloys according to their resistance to corrosion. Dent Mater. 2007. 23:1428–1437.
16. Benatti OF, Miranda WG Jr, Muench A. In vitro and in vivo corrosion evaluation of nickel-chromium- and copper-aluminum-based alloys. J Prosthet Dent. 2000. 84:360–363.
17. Rao SB, Chowdhary R. Evaluation on the corrosion of the three Ni-Cr alloys with different composition. Int J Dent. 2011. 2011:397029.
18. Geis-Gerstorfer J, Weber H. In vitro corrosion behavior of four Ni-Cr dental alloys in lactic acid and sodium chloride solutions. Dent Mater. 1987. 3:289–295.
19. Wylie CM, Shelton RM, Fleming GJ, Davenport AJ. Corrosion of nickel-based dental casting alloys. Dent Mater. 2007. 23:714–723.
20. Goodall TG, Lewis AJ. The metallography of heat treatment effects in a nickel-base casting alloy. A preliminary report. Aust Dent J. 1979. 24:235–237.
21. Lewis AJ. Metallographic changes and phase identification in a nickel base alloy upon fusion and casting. Aust Dent J. 1975. 20:378–383.
22. Leung VW, Darvell BW. Artificial salivas for in vitro studies of dental materials. J Dent. 1997. 25:475–484.
23. Reclaru L, Meyer JM. Zonal coulometric analysis of the corrosion resistance of dental alloys. J Dent. 1995. 23:301–311.
24. Holland RI. Corrosion testing by potentiodynamic polarization in various electrolytes. Dent Mater. 1992. 8:241–245.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download