Abstract
PURPOSE
This study was performed to define attachment and growth behavior of osteoblast-like cells and evaluate the gene expression on zirconia compared to titanium.
MATERIALS AND METHODS
MC3T3-E1 cells were cultured on (1) titanium and (2) zirconia discs. The tetrazolium-based colorimetric assay (MTT test) was used for examining the attachment of cells. Cellular morphology was examined by scanning electron microscopy (SEM) and alkaline phosphatase (ALP) activity was measured to evaluate the cell differentiation rate. Mann-Whitney test was used to assess the significance level of the differences between the experimental groups. cDNA microarray was used for comparing the 20215 gene expressions on titanium and zirconia.
RESULTS
From the MTT assay, there was no significant difference between titanium and zirconia (P>.05). From the SEM image, after 4 hours of culture, cells on both discs were triangular or elongated in shape with formation of filopodia. After 24 hours of culture, cells on both discs were more flattened and well spread compared to 4 hours of culture. From the ALP activity assay, the optical density of E1 cells on titanium was slightly higher than that of E1 cells on zirconia but there was no significant difference (P>.05). Most of the genes related to cell adhesion showed similar expression level between titanium and zirconia.
The success of dental implant is primarily based on good osseointegration, which depends on the biocompatibility of the implant material and implant surface properties, as well as on the quantity and quality of remaining tissue.1
Commercially pure titanium and titanium alloys are traditional materials used in most commercially available endosseous implants because of favorable mechanical properties and excellent biocompatibility.2 And titanium has been proven to be safe and efficient as an implant material in many studies.3,4 But recently, the biostability of titanium is increasingly questioned. Corrosion products in inner organs5 and galvanic side effects6 have been reported while using titanium implants. And allergic reactions and sensitivities to titanium have been also reported.7 Esthetic problems may arise due to dark metallic color of titanium when the thickness of soft tissue is insufficient to mask it and soft tissue resorption is expected. Long-term stability of esthetic implant restorations in anterior dentition is a challenge due to thin buccal bone and gingiva. While the survival rate and function of implant were main issues in implant dentistry in the past, nowadays the esthetic aspect of it became more focused. The success rate of the anterior maxillary implants was lower than other zones of the dental arch due to esthetic failure, while the survival rate of it showed no significant difference.8 Thus, tooth-colored materials have been considered as an implant fixture materials and ZrO2 might be the choice of material as the tooth-colored implant.
Zirconia is a bioinert nonresorbable material that has good chemical and dimensional stability, and a high bending strength and fracture toughness.9 Covacci et al. tested the potential toxicity of zirconia and the cell capability to grow and adhere upon the ceramic surfaces, and reported that zirconia showed good cytocompatibility and had no mutagenic and carcinogenic effects on cells.10 Zirconia shows less accumulation of plaque than titanium, and results in less irritation through the so-called bio-film. Rimondini et al. and Scarano et al. reported the low potential for bacterial colonization of zirconia.11,12 Much study on the biocompatibility and mechanical properties of zirconia has been done and zirconia has come to be used commercially as implant fixture materials. However, the gene expression levels between titanium and zirconia ceramic related to osteoblast adhesion and signal transduction are unknown.
This study was performed to define attachment and growth behavior of osteoblast-like cells MC3T3-E1 cultured on zirconia discs by MTT assay, scanning electron microscope, and ALP activity assay, and evaluate the gene expression of MC3T3-E1 cells on zirconium oxide surfaces compared to titanium surface using the cDNA microarray.
Titanium discs (Dentium. Co., Ltd, Suwon, Korea) used for the cell culture were machined from grade 4 titanium alloy. The discs were prepared to be 10 mm diameter and 2 mm thick and used as the culture substrate in the control group (titanium disc group). Zirconia discs (LAVA™, 3M ESPE, St. Paul, MN, USA) of Y-TZP (yttria-stabilized tetragonal zirconia polycrystal) were prepared in 10 mm diameter and 2 mm thick (zirconia disc group). The surface roughness values for each group were measured using 3D-Interactive Display (Wyko NT8000, Veeco Instruments Inc., Plainview, NY, USA). Two individual measurements per disc were made on two discs from each group. Area of 640 µm × 480 µm was used for measurement. The surface of titanium discs (a) had an average roughness (Ra) value of 322 nm, while that of zirconia discs (b) had an average roughness (Ra) value of 5.09 nm (Fig. 1). Disc samples were rinsed twice in absolute alcohol and once in demineralized water in ultrasonic, before sterilization by autoclave.
MC3T3-E1 cells, osteoblast-like cells from rat calvaria were obtained (Korean Cell Line Bank, Seoul, Korea) and cultured in sterile cell culture plate containing alpha minimal essential medium. For all experiments, cells were cultured on discs placed in 24-well plates.
Cells were collected and seeded at a density of 1 × 105 cells/mL by using 0.1% trypsin, 0.02% EDTA in Ca++ and Mg++ -free Eagle's buffer for cell release. One set of wells contained sterile 24 discs of titanium, whereas another contained 24 discs of zirconia at 37℃ in 5% CO2 for 24 hours and the samples were moved to new dishes and media was added and the plated discs were cultured at 37℃ in 5% CO2 for 24 hours. All cell culture media were supplemented with 100 units/mL penicillin-G, 100 g/mL streptomycin, and 0.25 g/mL fungizone (Gemini Bio-Products, Inc., Woodland, CA, USA). Total RNA extraction was performed with Qiagen mini kit (Qiagen, Chatsworth, CA, USA) for microarray assay.
Surfaces were analyzed using SEM to determine their microtopographic properties. The discs were viewed at magnifications of ×300 (before cell culture). Cell suspension (1 × 105 cell/mL) was seeded to titanium discs and zirconia discs. The cultures were incubated for 4 and 24 hours to evaluate the influence of the substrate geometry on titanium and zirconia using SEM. At the end of the various incubation times, the non-attached cells on the different substrates were removed by rinsing twice with 0.1 M sodium cacodylate buffered solution, dehydrated in a series of ethanol, dried by tetramethylsilane (Merck, Darmstadt, Germany) and sputter-coated with Au-Pd (Bio Rad, SC-500, Hertfordshire, UK). Finally, they were observed in a SEM (JSM-6430F, JEOL, Tokyo, Japan) at an accelerating voltage of 15kV. Three runs of experiments were carried out, which included all different samples in threefold.
The MTT test assay (Sigma, St. Louis, MO, USA) was used for examining attachment of cells after culturing the cells on titanium discs and zirconia discs for 4 hours and 24 hours. The substance used for MTT test was a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium salt, which turns into a blue formazan product due to the viable mitochondria in active cells. The cells were cultured on the 12 discs placed in 24-well culture plates and incubated at 37℃ in 5% CO2 for 4 hours and 24 hours. The discs were moved to new 24- well culture plates after 4 hours and 24 hours of incubation and new media was added. MTT solution (5 mg/mL) was added and incubated at 37℃ for 4 hours in 5% CO2. The media was discarded and 400 µL of isopropanol with 0.04 N HCl was added in each well. The product solution was moved into 96-well plates. The absorbance at 570 nm was measured using microplate reader (Bio-Rad, Hercules, CA, USA).
Cell suspension (1 × 106 cell/mL) was seeded to titanium discs and zirconia discs. The cultures were incubated for 4 days to evaluate ALP activity. After incubation times, culture media was removed by rinsing twice with phosphate buffered solution. The cells were added with 0.1% Triton X-100 (Polyscience, Warrington, PA, USA) and cultured for 30 minutes. Finally, the cells were added with 100 mM p-nitrophenyl-phosphate in 0.1 M glycine-NaOH (pH 10.4) and cultured for 10 minutes. The product solution was moved into 96 well plates. The absorbance at 409 nm was measured using microplate reader (Bio-Rad, Hercules, CA, USA).
The cDNA microarray Agilent Rat 22K chip (Digital Genomics, Seoul, Korea) was used to monitor the expression of 21575 genes. The samples (10 µg total RNA per condition) were processed according to the manufacturer's recommendation. Fluorescent-labeled cDNA for oligo microarray analysis was prepared by amplification of total RNA in the presence of aminoallyl-UTP followed by the coupling of Cy3 or Cy5 dyes (Amersham Pharmacia, Uppsala, Sweden). RNA extracted from cells grown on titanium discs was labeled with Cy3 and used as control against the Cy5-labeled RNA extracted from cells grown on zirconia discs in the first experiment and then switched.
Oligo microarray kit was hybridized with the fluorescently labeled RNA at 60℃ for 16 hours and then washed. This microarray contains 21575 distinct sequences (1075 control spots and 20500 gene spots). DNA chips were scanned using GenePix 4000B (Axon Instruments, Union City, CA, USA). Scanned images were analyzed with GenePix Pro 3.0 software (Axon Instruments, Union City, CA, USA) to obtain gene expression ratios. Gene expression ratios were normalized by LOWESS regression.
Test mean values and standard deviation (SD) were computed for MTT test and ALP activity assay. Nonparametric test for two group comparison by Mann-Whitney test was used to assess the significance level of the differences between the experimental groups. All statistical analyses were performed using SPSS software (Version 12.0, SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant for P values <.05.
The general shape and growth pattern of the osteoblast-like cells MC3T3-E1 was observed using SEM for each group. Fig. 2 and Fig. 3 shows representative SEM images of MC3T3-E1 cells cultured for 4 hours and 24 hours on titanium discs and zirconia discs. After 4 hours of culture, cells on both discs were irregularly triangular or elongated in shape with formation of filopodia that means good attachment to the substrate. After 24 hours of culture, cells on both discs were observed to be more flattened and well spread. Cells cultured on both discs showed no difference in cell density and morphology.
The density of MC3T3-E1 cells was measured over 2 different time-periods (4 hours and 24 hours) by using the MTT assay. Fig. 4 shows the optimal density of formazan produced by the MC3T3-E1 cells on zirconia and titanium surfaces. After 4 hours of adhesion, the osteoblast-like cell density on titanium surfaces and zirconia surfaces showed no significant difference (P>.05). After 24 hours of adhesion, the optical density of both groups significantly increased but there was no significant difference between both groups (P>.05).
After incubation of cells for 4 days, ALP activity was measured. The optical density of cells on titanium discs was slightly higher than that of cells on zirconia discs but there was no significant difference (P>.05). Fig. 5 shows the optimal density of the differentiation rate of the cells on zirconia and titanium surfaces.
After 24 hours, total of 20215 gene expressions were observed using cDNA microarray. Up regulated genes were classified as cDNA clones that exhibited a 2-fold or greater change in expression level. Upregulation means increased production of specific gene products, such as protein or RNA. Downregulated genes were categorized by the same criteria in reverse. The up- and down-regulated genes in zirconia surface are reported in Table 1 and Table 2.
When an implant is inserted into bone, it is expected that an apposition of bone to the implant surface will occur, a process called osseointegration.13 For this osteoinductive event, gaps between bone and implant must be sealed, and damaged bone by preparation of implant site must be repaired. The initial responses on implant in the bone are comprised of two aspects: the response of the host to the implant and the behavior of the material in the host. The almost immediate event that occurs upon implantation of biomaterials, is adsorption of proteins.14 This biological protein layer formed on the surface of implant biomaterials is believed to be responsible for host cell response. The ability of protein adsorption depends on the surface macro- and micro-topography, chemistry, and energy of implant biomaterials.15 The host response to implants placed in bone involves a series of cell and matrix events involving many physiologic, chemical and genetic pathways.16 This study emphasizes the cell response to implant biomaterials, especially zirconia ceramics.
There have been several studies that evaluate the biocompatibility of zirconia as implant material. Akagawa et al. and Albrektsson et al. reported that direct bone apposition was observed at bone-zirconia interfaces in histological and ultrastructural studies, and suggested that zirconia may also be a suitable implant material.17,18 Ko et al. investigated the initial osteoblast-like HOS cell response to yttrium-stabilized tetragonal zirconia polycrystal (Y-TZP) and reported that Y-TZP showed at least equivalent or slightly better biological response of osteoblast-like HOS cells than pure titanium during a short-time cell culture period.19
In our experiment, MTT assay and SEM examination showed that cellular attachment and proliferation were comparable between titanium and zirconia on a short-time cell culture period, which suggests that zirconia implants may be as favorable as titanium implants in attachment of osteoblasts on initial healing period after implant placement.
ALP activity is an important parameter typically used as markers of osteoblastic differentiation. In our experiment, the differentiation rate of E1 cells on cultured titanium and zirconia discs was similar each other.
We used the cDNA microarray technique for identifying the expression of important genes in cells adhesion, signal transduction, and transcription, based on previous literatures. Cell adhesion to materials play important roles in biological processes including cell motility, cell proliferation, cell differentiation, regulation of gene expression and cell survival. There are three major parts, involved in cell adhesion to materials; extracellular matrix (ECM) proteins, cytoskeletal molecules, cell adhesion molecules (CAMs).
Some ECM proteins like fibronectin, osteopontin, bone sialoprotein, thrombospondin, type I collagen, vitronectin have chemotactic or adhesive properties due to their Arg-Gly-Asp (RGD) sequence which is specific to the fixation of cell transmembrane receptors like integrin.20 In our experiment, fibronectin type III, type I collagen, and vitronectin showed similar expression levels between titanium and zirconia.
At the site of contact between tissue-cultured cell and extracellular matrix, absorbed onto substrate surface, specialized structures, so-called "focal contacts" or "adhesion plaque", are formed. This adhesions are closed junctions where the distance between the substrate surface and the cell membrane is about 10-15 nm.21 On the intracellular part, cytoskeletal proteins are involved in signal transduction with integrin, proteases, protein kinases, kinase phosphatases, and other signaling molecules.22 Among the genes related to regulation of actin cytoskeleton, integrin α8 (ITGA8) were upregulated, while protein tyrosine kinase 2 (PTK2), integrin α3 (ITGA3) were downregulated in zirconia.
Integrins are integral cell-surface proteins, which are known to participate in cell-surface adhesion, as well as cell-surface mediated signaling.23 Integrin-mediated adhesion is a highly regulated process involving receptor ligand interactions and subsequent adhesion strengthening and cell spreading. In our experiment, integrin α8 (ITGA8) was upregulated, while integrin α3 (ITGA3), α5 (ITGA5) were downregulated in zirconia. It was reported that adhesion to fibronectin was inhibited by antibodies to the ITGA5.24
Among other genes involved in focal adhesion, laminin α1, α5, β1 subunit 1 were down-regulated in zirconia. Laminin is a major non-collagenous protein of the basal lamina and provides excellent conditions for the promotion of neurite outgrowth.25
Among the cell adhesion molecules (CAMs) observed in this experiment, thiopurine methyltransferase (TPMT), an enzyme that methylates thiopurine compounds, and CD6 antigen (CD6), surface molecule that may regulate CD5 tyrosine phosphorylation, were downregulated in zirconia. Syndecan 1 (SDC1), a member of the syndecan proteoglycan family, was also downregulated in zirconia. The syndecans mediate cell binding, cell signaling, and cytoskeletal organization.26
Tight junctions (or zonula occludens) are composed of transmembrane protein and a cytoplasmic "plaque" consisting of many different proteins that form large complexes. The transmembrane proteins mediate cell adhesion and constitute the intramembrane and paracellular diffusion barriers.27 Tight junction protein complex, consist of cytoplasmic "plaque" of tight junctions, appears to organize the transmembrane proteins and couple them to other cytoplasmic proteins and to actin microfilaments.28 In our experiments, upregulation of tight junction protein 1 (TJP1) was observed.
Protein tyrosine phosphatases (PTPs) may promote the disassembly or turnover of focal contact and regulate the phosphorylation state of many signalling molecules, such as the mitogen-activated protein (MAP) kinase family.29 The expression of most of PTPs showed similar expression level between titanium and zirconia., while protein tyrosine phosphatase, receptor type, F polypeptide (PTPRF), interacting protein, α1, protein tyrosine phosphatase receptor type, M (PTPRM), and protein tyrosine phosphatase receptor type, U (PTPRU) were downregulated in zirconia.
Growth hormone receptor (GHR) was up regulated, while transforming growth factor, leukemia inhibitory factor (LIF), and fibroblast growth factor were down regulated in zirconia.
Using cDNA microarray, total of 20215 gene expressions were observed and only 147 genes were up regulated and 838 genes were down regulated in zirconia. However, most genes related to cell adhesion showed similar expression level between titanium and zirconia, which correlated with the results of SEM and MTT assay. Also, most genes related to signal transduction including the activation of several pathways showed similar expression level between titanium and zirconia. Thus, these results suggested that zirconia ceramics could regulate genetic effect of osteoblast-like cells, as titanium did.
This study has reported on the influence of implant material on gene expression. Recently, surface treatments, like the change of surface topography, chemistry, and material composition, are on trial to improve cell adhesion and osteoinductive potential on titanium implants. Further study is necessary to investigate biological response of cells on zirconia surface with various surface treatments.
Zirconia ceramic showed comparable biological responses of osteoblast-like cells to titanium during a short-time cell culture period. Most of the genes related to cell adhesion and signal transduction including the activation of several pathways showed similar expression level between titanium and zirconia. This suggests that zirconia implants could be as favorable as titanium implants in attachment of osteoblasts on initial healing period after implant placement.
Figures and Tables
 | Fig. 1Surface topography of the (A) titanium disc, and (B) zirconia disc. Titanium discs showed greater surface roughness than zirconia discs. |
 | Fig. 2SEM image of osteoblasts cultured on zirconia and titanium surfaces at 4 hours (×300). (A) titanium group, (B) zirconia group. Single randomly oriented cells were covering the surface on both substrates. The cells cultured on titanium and zirconia discs were irregularly triangular or elongated in shape showing similar cellular morphology. |
 | Fig. 3SEM image of osteoblasts cultured on zirconia and titanium surfaces at 24 hours (×300), (A) titanium group, (B) zirconia group. The cells on both specimens are connected to each other. |
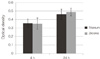 | Fig. 4Evaluation of cellular attachment by using MTT assay after 4 and 24 hours of incubation of osteoblasts. The osteoblast-like cell density on titanium and zirconia surfaces showed no significant difference (P>.05). |
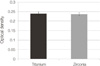 | Fig. 5Evaluation of the cellular differentiation by using ALP activity test after 4 days incubation of osteoblasts. The ALP activity of osteoblasts on zirconia was similar to that of osteoblasts on titanium (P>.05). |
References
1. Røynesdal AK, Ambjørnsen E, Støvne S, Haanaes HR. A comparative clinical study of three different endosseous implants in edentulous mandibles. Int J Oral Maxillofac Implants. 1998; 13:500–505.
2. Viornery C, Guenther HL, Aronsson BO, Péchy P, Descouts P, Grätzel M. Osteoblast culture on polished titanium disks modified with phosphonic acids. J Biomed Mater Res. 2002; 62:149–155.
3. Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990; 5:347–359.
4. van Steenberghe D. A retrospective multicenter evaluation of the survival rate of osseointegrated fixtures supporting fixed partial prostheses in the treatment of partial edentulism. J Prosthet Dent. 1989; 61:217–223.
5. Schliephake H, Reiss G, Urban R, Neukam FW, Guckel S. Metal release from titanium fixtures during placement in the mandible: an experimental study. Int J Oral Maxillofac Implants. 1993; 8:502–511.
6. Cortada M, Giner L, Costa S, Gil FJ, Rodríguez D, Planell JA. Galvanic corrosion behavior of titanium implants coupled to dental alloys. J Mater Sci Mater Med. 2000; 11:287–293.
7. Yamauchi R, Morita A, Tsuji T. Pacemaker dermatitis from titanium. Contact Dermatitis. 2000; 42:52–53.
8. Henry PJ, Laney WR, Jemt T, Harris D, Krogh PH, Polizzi G, Zarb GA, Herrmann I. Osseointegrated implants for singletooth replacement: a prospective 5-year multicenter study. Int J Oral Maxillofac Implants. 1996; 11:450–455.
9. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999; 20:1–25.
10. Covacci V, Bruzzese N, Maccauro G, Andreassi C, Ricci GA, Piconi C, Marmo E, Burger W, Cittadini A. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials. 1999; 20:371–376.
11. Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002; 17:793–798.
12. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004; 75:292–296.
13. Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999; 20:2311–2321.
14. Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996; 17:137–146.
15. Chesmel KD, Clark CC, Brighton CT, Black J. Cellular responses to chemical and morphologic aspects of biomaterial surfaces. II. The biosynthetic and migratory response of bone cell populations. J Biomed Mater Res. 1995; 29:1101–1110.
16. Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999; 20:2311–2321.
17. Akagawa Y, Ichikawa Y, Nikai H, Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993; 69:599–604.
18. Albrektsson T, Hansson HA, Ivarsson B. Interface analysis of titanium and zirconium bone implants. Biomaterials. 1985; 6:97–101.
19. Ko HC, Han JS, Bächle M, Jang JH, Shin SW, Kim DJ. Initial osteoblast-like cell response to pure titanium and zirconia/alumina ceramics. Dent Mater. 2007; 23:1349–1355.
20. Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994; 9:487–496.
21. Burridge K, Fath K. Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. Bioessays. 1989; 10:104–108.
22. Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992; 119:893–903.
23. Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997; 9:76–85.
24. Pistone M, Sanguineti C, Federici A, Sanguineti F, Defilippi P, Santolini F, Querzé G, Marchisio PC, Manduca P. Integrin synthesis and utilization in cultured human osteoblasts. Cell Biol Int. 1996; 20:471–479.
25. Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000; 218:213–234.
26. Jaakkola P, Jalkanen M. Transcriptional regulation of Syndecan-1 expression by growth factors. Prog Nucleic Acid Res Mol Biol. 1999; 63:109–138.
27. Wongdee K, Pandaranandaka J, Teerapornpuntakit J, Tudpor K, Thongbunchoo J, Thongon N, Jantarajit W, Krishnamra N, Charoenphandhu N. Osteoblasts express claudins and tight junction-associated proteins. Histochem Cell Biol. 2008; 130:79–90.
28. Willott E, Balda MS, Heintzelman M, Jameson B, Anderson JM. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992; 262:C1119–C1124.
29. Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004; 117:699–711.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download