Abstract
PURPOSE
The aim of this study was to evaluate the surface properties and in vitro bioactivity to osteoblasts of magnesium and magnesium-hydroxyapatite coated titanium.
MATERIALS AND METHODS
Themagnesium (Mg) and magnesium-hydroxyapatite (Mg-HA) coatings on titanium (Ti) substrates were prepared by radio frequency (RF) and direct current (DC) magnetron sputtering.The samples were divided into non-coated smooth Ti (Ti-S group), Mg coatinggroup (Ti-Mg group), and Mg-HA coating group (Ti-MgHA group).The surface properties were evaluated using scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). The surface roughness was evaluated by atomic force microscopy (AFM). Cell adhesion, cell proliferation and alkaline phosphatase (ALP) activity were evaluated using MC3T3-E1 cells. Reverse transcription polymerase chain reaction (RT-PCR) analysis was performed.
RESULTS
Cross-sectional SEM images showed that Mg and Mg-HA depositionson titanium substrates were performed successfully. The surface roughness appeared to be similaramong the three groups. Ti-MgHA and Ti-Mg group had improved cellular responses with regard to the proliferation, alkaline phosphatase (ALP) activity, and bone-associated markers, such as bone sialoprotein (BSP) and osteocalcin (OCN) mRNA compared to those of Ti-S group. However, the differences between Ti-Mg group and Ti-MgHA group were not significant, in spite of the tendency of higher proliferation, ALP activity and BSP expression in Ti-MgHA group.
Titanium (Ti) alloys are extensively used in orthopaedic and dental implants due to corrosion resistance, biocompatibility, machinability and load bearing capability. In order to improve the biocompatibility, several surface treatment and coating methods have been developed. Hydroxyapatite (HA) coating on titanium substrate is desirable for biomedical applications owing to its bone forming ability.1 Recently, magnesium (Mg) alloys or Mg-coated surfaces on metallic substrates wereintroduced in medical applications.2,3 A few studies have reported an increase in the attachment and functions of osteoblast on the Mg surface.4,5 On the other hand, there are some concerns regardingthefast degradation of Mg as a response to the body fluid. Mg dissolution leads to an increase in pH, reducingthe biological activities of osteogenic cells. Therefore, several studies have been performed to strengthen the Mg surface with coatings and surface treatments.2,6 They showed that the calcium-phosphate coating provided magnesium with a good surface bioactivity and increased new bone formation at the implant/bone interface. Therefore, a surface coating is an effective method for reducing the drawbacks of magnesium and improving the surface bioactivity.
The aim of this study was to evaluate the surface properties and in vitro bioactivity to osteoblasts of magnesium (Mg) and magnesium-hydroxyapatite (MgHA) coated titanium compared to non-coated titanium. In the present study, Mg and MgHA coating layers were developed on the Ti substrates by radio frequency (RF) and direct current (DC) magnetron sputtering, while in other studies electrochemical deposition was used to develop Mg coating layers.
Synthetic HA (Ca10(PO4)6(OH)2) disc (CERAC Co., USA, 127 mm diameter, 10 mm thickness) and pure Mg metal disc (127 mm diameter, 5 mm thickness, 99.9% purity) were used as the target. The Ca/P atomic ratio of HA disc was approximately 1.68.
Commercially pure machined titanium (grade II) discs were prepared (12 mm and 25 mm diameter, 1 mm thickness). The discs were wet ground with 240, 400, and 600 grit silicon carbide paper, and then cleaned ultrasonically in acetone and ethanol for 10 minutes each, with deionized water rinsing between applications of each solvent.
RF magnetron sputtering was carried out using L-210HS-F (Anelva Corp., USA). Mg deposition was performed by DC sputtering for 10 minutes. A 127 mm-diameter high purity Mg (99.99%) target was mounted parallel to the substrate, at a distance of 47 mm in the deposition chamber. The sputtering chamber was evacuated to a pressure <1x = 10y5 Pa using an oil diffusion pump with a liquid nitrogen trap. Argon gas (99.999%) was then introduced into the chamber using a mass flow controller (66.7 Pa). The HA films were grown in a vacuum at room temperature on single side of the synthetic HA disc. The HA deposition was performed by RF magnetron sputtering for 5 hours (100W). The inlet flow rate of the sputtering gas, argon (Ar), was kept at 100 sccm. The base pressure of the systemwas 3 × 10-4 Pa. During deposition, the Ar flow rate was fixed at 120 sccm and the O2 flow rate was varied from 0 to 30 sccm, which caused a change in the total pressure in the chamber in the range from 1.5 to 1.7 Pa. The total pressure was kept constant during deposition. After coating, the samples were divided into 3 groups. Group Ti-S was a non-coated smooth Ti surface. Group Mg was a Ti surface coated with Mg by DC sputtering. Group Ti-MgHA was a Ti surface coated with Mg by DC sputtering and HA by RF magnetron sputtering.
Scanning electron microscopy (SEM, S-4700, Hitachi, Japan) wasused to evaluate the surface morphology. The surface composition was examined by X-ray photoelectron spectroscopy (XPS.MULTILAB2000SYSTEM, SSK Co., USA). Surface roughness (Rrms: root-mean-square roughness) was evaluated by atomic force microscopy (AFM, Nano Scope IIIa, Digital Instrument, USA).
Mouse MC3T3-E1 cells (ATCC, Rockville, MD) were cultured in T-75 flasks in alpha minimum essential medium (α-MEM, Invitrogen Co., USA) supplemented with 10% heat-inactivated fetal bovine serum, 100 mg/mL penicillin, and 100 mg/mL streptomycin (Invitrogen Co., USA) at 37℃ in humidified atmosphere of 5% CO2-95% air.
MC3T3-E1 cellswere evaluated by SEM for cell attachment and growth. The cells were seeded in a 12 well plate at a density of 1 × 104 cells/mL in α-MEM. After incubation for two days, the dishes were washed three times with phosphate buffered saline and fixed with 2.5% glutaraldehyde in 100 mM cacodylate buffer. The samples were dehydrated in increasing concentrations of ethanol, air-dried, and mounted on aluminum stubs and coated with platinum.
The MC3E3-T1 cells were cultured on Ti-S, Ti-Mg and Ti-MgHA surfaces (12 mm diameter) in 12 well plates at a density of 1 × 104 cells/mL in α-MEM. After incubation, cell proliferation was assessed using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96 AQueous, Promega, USA). Formazan accumulation was quantified by the absorbance at 490 nm using an enzyme-linked immunoabsorbant assayplate reader (Microplate manager, BIO-RAD, USA).
To measure the ALP activity, MC3T3-E1 cells were seeded on Ti-S, Ti-Mg and Ti-MgHA discs (12 mm diameter) in a 12 well plate at a density of 1 × 104 cells/mL in α-MEM. The ALP activity was determinedon day 7. Briefly, the cells were lysed in Triton 0.1% (Triton X-100) in PBS, frozen at -70℃ and then thawed. 100 µL of the cell lysates was mixed with 200 µL of 10 mM p-nitrophenol phosphate and 100 µL of 1.5 M 2-amino-2-methyl-1-propanol buffer, and then incubated for 60 minutes in an oven at 60℃. The ALP activity was measured from the absorbance reading at 405 nm with a spectrophotometer (SmartSpec, BIO-RAD, USA).
All sample discs (25 mm diameter) were placed in the 6 well tissue culture dishes under aseptic conditions. Subsequently, 1.0 × 105 cells/mL in α-MEM were seeded into each well and incubated for 7 days. The total RNA was isolated on day 7 using the methodology described by the manufacturer. PCR was then performed using amplication primer sets (Sigma-Genosys, USA) for bone sialoprotein (BSP, 1068 base pairs (bp): forward, 5'-AACAATCCGTGCCACTCA-3'; reverse, 5'-AACAATCCGTGCCACTCA-3'), collagen type-I (COL-I, 250 bp: forward, 5'-TCTCCACTCTTCTAGGTTCCT-3'; reverse, 5'-TTGGGTCATTTCCACATGC-3'), osteocalcin (OCN, 198bp: forward, 5'-TCTGACAAACCTTCATGTCC-3'; reverse, 5'-AAATAGTGATACCGTAGATGCG-3') and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 418 bp: forward, 5'-CACCATGGAGAAGGCCGGGG-3'; reverse, 5'-GACGGACACATTGGGGGTAG-3') genes. A semi-quantitative comparison with GAPDH was performed to assess the changes in BSP, COL-I and OCN gene expression in the titanium specimens using a Gel-Doc imaging system (BIO-RAD, USA). These experiments were performed in duplicate and 2 samples per group were used for each experiment.
SEM images showed that uniform Mg and Mg-HA layerswith relatively well-developed columnar structures were developed on the Ti surface (Fig. 1). The thickness of Mg and Mg-HA layer were 11 and 20 µm, respectively, as shown in the cross-sectional images (Fig. 1B and Fig. 1D). The surface chemical composition of Ti-Mg and Ti-MgHA was analyzed by XPS (Fig. 2). The main element peaks of Ti-S were Ti and O. Ti-Mg showed Mg peak, while Ti-MgHA showed Ca and P peaks in addition to Mg peak.
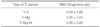
Profile roughness measurements revealed 0.24, 0.25, and 0.25 µm of root mean square (RMS) roughness in Ti-S, Ti-Mg and Ti-MgHA, respectively (Table 1). The surface roughness appeared to be similar in all groups.
For each specimen, the cell morphology was examined by SEM after 48 h of cell seeding. In this study, MC3T3-E1 cells adhered tightlyto the surfaces of the Ti-S, Ti-Mg and Ti-MgHA surfaces. The cells were spread extensively, flattened with elongated shapes and connected to adjacent cells by filopodia (Fig. 3). No morphological difference was observed between Ti-S, Ti-Mg, and Ti-MgHA.
Cell proliferation was measured by aMTS assay (Fig. 4). On day 3, the Ti-Mg and Ti-MgHA surfaces showed a proliferation rate of 104% to 107%, respectively, compared to Ti-S (P>.05). On day 5, the optical densities on the three investigated surfaces were significantly higher than those observed on day 3 (P<.05). On day 5, Ti-Mg and Ti-MgHA showed a proliferation rate of 112% to 124%, respectively, compared to Ti-S (P>.05). The cells on Ti-Mg and Ti-MgHA showed 50-60% higher ALP levels than those on Ti-S (P<.05, Fig. 5)
Fig. 6 compares the patterns BSP, COL-I, and OCN mRNA expression of the cells cultured on Ti-Mg, Ti-MgHA, and Ti-S at 7 days. The cells on Ti-S, Ti-Mg, and Ti-MgHA expressed the COL-I gene well. The cells on Ti-Mg and Ti-MgHA increased mRNA expressions of BSP and OCN more than the cells on Ti-S. BSP mRNA expression on Ti-Mg and Ti-MgHA increased approximately 1.8-fold and 2.1-fold respectively. OCN mRNA expression on Ti-Mg and Ti-MgHA increased approximately 1.5-fold and 1.4-fold, respectively (Fig. 6).
The main element peaks of Ti-S were Ti and O. Ti-Mg showed Mg peak while Ti-MgHA showed Ca and P peaks in addition to Mg peak. Because Ca and P are elements of HA (Ca10(PO4)6(OH)2), these results imply Mg and HA layers were well developed on the Ti substrate.
No obvious morphological difference was observed amongcells attached on Ti-S, Ti-Mg, and Ti-MgHA surfaces. Cell proliferation measurement presented a tendency that Ti-Mg and Ti-MgHA surfaces showed higher proliferation rate than Ti-S, although the difference was not significant. In addition, the difference between Ti-Mg and Ti-S or between Ti-MgHA and Ti-S increased with time.
Surface roughness is one of the factors influencing on cell responses.7 However, both coating groups and Ti-S showed little difference in surface roughness in the present study, while surface composition showed a clear difference. Therefore, it can be suggested that the result of cell proliferation measurement in Ti-Mg and Ti-MgHA surfaces might be attributed to the different surface chemistry.
Although there was no significant difference among the groups, the Ti-Mg and Ti-MgHA surface tended to showa higher proliferation level than Ti-S and Mg-HA coating tended to enhance cell proliferation more than Mg coating. Surface modification with Mg was reported to increase the level of osteoblastic cell adhesion and proliferation, which were associated with intergrin receptors.8 Ibasco et al.3 also reported that calcium-phosphatecoating formed on a Mg coated Ti surface enhanced the osteoblast viability up to 8 days. This can explain why the Ti-Mg and Ti-MgHA surface in the present study showeda higher proliferation level with respect to the Ti-S surface. Sampaio et al.9 and Hashimoto et al.10 showed osteoblasts grow faster on high-crystallinity HA coatings than uncoated Ti surface. The calcium-phosphatelayers on the Ti substrate also enhance cell attachment and proliferation.
Regarding coating methods, we mainly focused on the releasing ion from the coatings, so post-deposition heat treatment was not performed. Hulshoff et al.11 have suggested thatmagnetron-sputtered Ca-P coatings show the similar result of bone healing as the plasma-sprayed Ca-P coatings and there was little difference in amount of bone between heat-treated coatingand amorphous coating at 9 weeks. Due to the difference of the thermal expansion coefficients of titanium and Ca-P coating, post deposition heat treatment causes delamination of Ca-P coating, leading to the failure of implants clinically. Dissolution of amorphous coatingmay promoteprecipitation of Ca-P apatite layer from the locally supersaturated fluids. Barrère et al.12 described that the Ca-P deposition on titanium surface is needed chemicalbonding of nanosized clusters and stabilized bymagnesium ions. Mg ion also promotes the adherence of the precipitated Ca-P apatite layers.
The osteoblastic cells initially increase their number and produce an extracellular matrix. The differentiation phase then follows, which is characterized by the high levels of alkaline phosphatase (ALP) production and modifications of the matrix.13 In this study, the cells on Ti-Mg and Ti-MgHA after 7 days showed 50 and 60% higher ALP levels than those on Ti-S, respectively (P<.05). This implies that the surface chemical compositionsappear to affect the ALP activity, indicating the facilitation of osteoblastic differentiation. Increased ALP activity shows the increase in cell differentiation onto the chemical modification of Ti substrate.
Bone-associated markers, such as bone sialoprotein (BSP) and osteocalcin (OCN) were evaluated by semi-quantitative RT-PCRto evaluate the effect of Mg and MgHA coating on bone-associated markers. COL-I is an essential component of the extracellular matrix that is required before mineralized matrix formation. In this study, the cells on Ti-S, Ti-Mg, and Ti-MgHA expressed the COL-I gene well. BSP and OCN are secreted by osteoblasts and regulate mineralization and maturation.14 Thehigher levels of BSP and OCNmRNA expression with increased ALP activity indicated a more differentiated phenotype for the MC3T3-E1 on Ti-Mg and Ti-MgHA, compared to the cells on Ti-S.
Zreiqat et al.5,8 examined the osteogenic response to an Mg-ion-modified metal surface and reported enhanced osteoblastic differentiation. The improved osteogenic response contributes to the increased expression of integrin receptors of Mg-ion-modified metal surface. The integrin receptors were reported to increase the level of cell proliferation and promote the osteoblastic differentiation of bone cells. A HA film coated on biomaterials also increased the level of osteoblastic cell proliferation and differentiation, which was associated with a high level of fibronectin receptors.14 In this study, MC3T3-E1 cells were closely attached to the coated Ti surface and Mg and HA might facilitate integrin-mediated proliferation and differentiation.
Mg corroded too rapidly in vivo. To improve the corrosion resistance of Mg, many studies regarding different types of coating and surface treatement were performed.2,6 Magnetron sputtering was used to produce homogeneous and dense crystalline HA coating.15 Inthis study, a magnesium-hydroxyapatite coating surface had a dense and high crystalline structure, which might inhibitthe rapid dissolution of Mg. Consequently,the slowed dissolution of Mg on the Ti substrate preventedan abrupt change in pH and Mg concentration inthe culture media. Our pilot study showed that the Ti-MgHA surface in distilled water for 30 days still contained Mg, Ca, and P elements on the surface, but Ti-Mg did not contain Mg on the surface. Therefore, a Mg HA coating mightreduce the dissolution rate of Mg and effectively enhance the osteogenic response. On the other hand, the experimental time period in this study was up to 7 days, which might be short for evaluating the response of osteoblasts to Mg and Mg HA coated Ti surface. Further in vivo and in vitro studies will be needed to determine the stability and other biological features of Ti with Mg HA coatings.
In addition, the present study did not compare the stability of coating layers developed by magnetron sputtering and other coating methods. However, the thicknesses of coating layers were higher than reported in other studies.
In summary, Mg and Mg HA coating by RF and DC magnetron sputtering was found to stimulate differentiation into osteoblasts of MC3T3-E1 cells, potentially contributing to rapid osseointegration.HA coating on the Ti-Mg surface might be helpful for protection of Mg coating by forming a dense and high crystalline layer to be hardly degraded.
Figures and Tables
 | Fig. 1Scanning electron microscopy (SEM)images of (A and B) Ti-Mg and (C and D)Ti-MgHA. ((A and C) Overview, (B and D) Cross-sectional view). |
 | Fig. 3SEM images of cell adhesion on the titanium surfaces at 2 days. MC3T3-E1 cells on (A) Ti-S, (B) Ti-Mg surface, and (C) Ti-Mg-HA surface. |
References
1. Porter AE, Taak P, Hobbs LW, Coathup MJ, Blunn GW, Spector M. Bone bonding to hydroxyapatite and titanium surfaces on femoral stems retrieved from human subjects at autopsy. Biomaterials. 2004; 25:5199–5208.
2. Xu L, Pan F, Yu G, Yang L, Zhang E, Yang K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials. 2009; 30:1512–1523.
3. Ibasco S, Tamimi F, Meszaros R, Nihouannen DL, Vengallatore S, Harvey E, Barralet JE. Magnesium-sputtered titanium for the formation of bioactive coatings. Acta Biomater. 2009; 5:2338–2347.
4. Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006; 27:1728–1734.
5. Zreiqat H, Howlett CR, Zannettino A, Evans P, Schulze-Tanzil G, Knabe C, Shakibaei M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J Biomed Mater Res. 2002; 62:175–184.
6. Lorenz C, Brunner JG, Kollmannsberger P, Jaafar L, Fabry B, Virtanen S. Effect of surface pre-treatments on biocompatibility of magnesium. Acta Biomater. 2009; 5:2783–2789.
7. Na Y, Heo SJ, Kim SK, Koak JY. Implant surface treatments affect gene expression of Runx2, osteogenic key marker. J Adv Prosthodont. 2009; 1:91–96.
8. Zreiqat H, Howlett CR, Zannettino A, Evans P, Knabe C, Schulze-Tanzil G, Shakiabei GM. Surface modification of bioceramics affect osteoblastic cells response. Key Eng Mater. 2003; 240-242:707–710.
9. Sampaio BV, Göller G, Oktar FN, Valério P, Goes A, Leite MF. Biocompatibility evaluation of three different titanium-hydroxyapatite composites. Key Engin Mater. 2005; 284-286:639–642.
10. Hashimoto Y, Kusunoki M, Hatanaka R, Hamano K, Nishikawa H, Hosoi Y, Hontsu S, Nakamura M. Improvement of hydroxyapatite deposition on titanium dental implant using ArF laser ablation: effect on osteoblast biocompatibility in vitro. Adv Sci Technol. 2006; 49:282–289.
11. Hulshoff JE, van Dijk K, van der Waerden JP, Wolke JG, Kalk W, Jansen JA. Evaluation of plasma-spray and magnetron-sputter Ca-P-coated implants: an in vivo experiment using rabbits. J Biomed Mater Res. 1996; 31:329–337.
12. Barrère F, van der Valk CM, Dalmeijer RA, Meijer G, van Blitterswijk CA, de Groot K, Layrolle P. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. J Biomed Mater Res A. 2003; 66:779–788.
13. Lian JB, Stein GS. The developmental stages of osteoblast growth and differentiation exhibit selective responses of genes to growth factors (TGF beta 1) and hormones (vitamin D and glucocorticoids). J Oral Implantol. 1993; 19:95–105.
14. El-Ghannam A, Ducheyne P, Shapiro IM. Porous bioactive glass and hydroxyapatite ceramic affect bone cell function in vitro along different time lines. J Biomed Mater Res. 1997; 36:167–180.
15. Wan T, Aoki H, Hikawa J, Lee JH. RF-magnetron sputtering technique for producing hydroxyapatite coating film on various substrates. Biomed Mater Eng. 2007; 17:291–297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download