Abstract
PURPOSE
The purpose of this study was to evaluate the effect of alendronates on bone remodeling around titanium implant in the maxilla of rats.
MATERIALS AND METHODS
The maxillary first molars were extracted and customized-titanium implants were placed immediately in thirty male Sprague-Dawley rats. The rats were divided into experimental (bisphosphonate) group and control group. At 4 weeks after implantation, the rats in the bisphosphonate group were subcutaneously injected with alendronate three times a week for 6 weeks where as the rats in control group were injected with saline. The rats were sacrificed at 1, 2, 3, 4, or 6 weeks after starting of injection and maxillary bones were collected subsequently. Alveolar bone remodeling around the implants were evaluated by radiographic and histologic analysis. Microarray analysis and immunohistomorphologic analysis were also performed on one rat, sacrificed at 6 weeks after starting of injection, from each group. Statistical analysis was performed using repeated measures analysis of variance and independent t test at a significance level of 5%.
RESULTS
There was no statistically significant difference in the bone area (%) around implant between the bisphosphonate group and the control group. However, the amount of empty lacuna was significantly increased in the bisphosphonate group, especially in the rats sacrificed at 4 weeks after starting of injection compared to that of the corresponding control group. The bisphosphonate group showed the same level of TRAP positive cell count, osteocalcin and angiopoietin 1 as the control group.
Bisphosphonates are drugs used most commonly in the management of bone metabolic abnormalities such as osteoporosis, Paget's disease and even cancer-associated bone disease.1 It is well known from many previous studies that bisphosphonates reduce bone resorption effectively by hindering the function of osteoclast as well as hydroxyapatite breakdown. Despite this high clinical efficacy of bisphosphonates, several complications associated with bisphosphonates therapy such as osteonecrosis of the jaw, atrial fibrillation, and hypocalcemia have been reported.1
Since Marx2 first had reported observing osteonecrosis in 36 patients who received intravenous bisphosphonates following dental treatment, many studies have been done to investigate contributing factors for bisphosphonate-related osteonecrosis of the jaw (BRONJ). In 2004, Ruggiero et al.3 suggested that not only intravenous bisphosphonates but also oral bisphosphonates could be a contributing factor for BRONJ. While the mechanisms of BRONJ have not yet been clarified, suppression of remodeling, impairment of angiogenesis as well as infection have been suggested as causative mechanisms.4
Among possible local risk factors for BRONJ including dental extraction, dental implant placement and periodontal surgery involving osseous injury, which are all suggested by American Association of Oral and Maxillofacial Surgeons, dental extraction has been one of the most frequently reported risk factors for BRONJ and this is supported by numerous studies published in the past 5 years.5,6 However, the use of bisphosphonates as a risk factor for developing BRONJ following implant therapy is not elucidated and still controversial despite increased use of dental implant these days.
Recently, several studies have reported that BRONJ is strongly associated with reduced bone turnover rate during bone remodeling phase which is a normal process taking place after a successful osseointegration. Allen and Burr4 insisted in their animal study with 3 years of daily oral bisphosphonate treatment that necrosis results from the suppression of bone matrix removal due to bisphosphonate-induced turnover suppression, assuming that there always is equilibrium between rate of bone matrix formation and removal under normal conditions. Lazarovici et al.7 have reported of the 11 patients, who received oral bisphosphonates, 6 patients developed BRONJ during the first 6 months after implant placement. Interestingly, BRONJ was also developed in 5 patients during bone remodeling phase. On the other hand, Grant et al.8 have reported that there was no evidence to support the effect of oral bisphosphonate on survival rate of implant. In their study, there was no significant difference in implant survival rate between patients who had received oral bisphosphonates and patients who had not received oral bisphosphonates. In addition to this controversy, most animal studies done regarding bone remodeling around dental implant was carried out using long bones such as tibia or femur,9,10 which may not reflect the effect of bisphosphonates on bone remodeling accurately. Since the overall rate of turnover of alveolar bone is 10 times greater than that of the long bones11 and the development and physiology of the jaw bones are different from other bones,12-14 the effect of bisphosphonate on bone remodeling is required to be investigated in jaw bones. Moreover, although numerous studies have reported the effect of bisphosphonates on immediate healing of implant site, not many studies have addressed the effect of bisphosphonates on remodeling of bone around implant site. Therefore, the purpose of this study was to evaluate the effect of bisphosphonate on bone remodeling around titanium implant in the maxilla of rats.
Thirty male Sprague-Dawley rats (body weight: 130-140 g; age: 4 weeks) were divided into the bisphosphonate group and control group and each group was subdivided into 5 groups having 3 rats in each groups according to duration of administration (1, 2, 3, 4 and 6 weeks). Animals were housed at the animal experimental laboratory at Yonsei University, College of Dentistry, Seoul, Korea and allowed free access to food pellets and tap water. All experiments were performed in accordance with the guidelines for animal experiments of Yonsei University College of Dentistry.
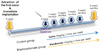
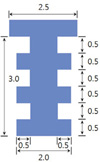
All surgical procedures involved in the study were conducted under general anesthesia with a mixture of Rumpun (xylazine, 20 mg/mL, 0.5 mL/kg body mass, Bayer, Leverkusen, Germany) and Zoletil (tiletamine and zolazepam, 100 mg/mL, 0.5 mL/kg body mass; Virbac, Carros, France) via intramuscular injection.15 After maxillary right first molar was extracted using hemostat, osteotomy was performed with a 1.8 mm diameter bur in the extraction sockets of the mesial roots (the largest root), followed by implantation of titanium mini-implant (ø 2.0 × 2.5 mm) (Fig. 2) until the top of the mini-implant reached the peripheral bone. Osseointegration was expected to occur within the first four weeks after implant placement. The rats in bisphosphonate group were then subcutaneously injected with 1.0 mg/kg of alendronates (Alendronate Sodium Salt, Merial Inc., Parramata, NSW, Australia) three times a week for 6 weeks whereas rats in control group were injected with saline. Rats were sacrificed administration by perfusion fixation with 4% paraformaldehyde at 1, 2, 3, 4 or 6 weeks after initial administration (Fig. 1).
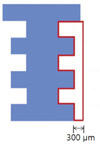
All specimens were decalcified with 10% EDTA at 4℃ for 2 months. Prior to embedding of the decalcified samples in paraffin wax, customized implant was removed and a series of 7 µm sections were then prepared. The sectioned specimens were stained with hematoxylin-eosin (H&E), Trichrome (Masson) using a kit (Sigma, St. Louis, MO, USA), and TRAP (tartrate-resistant acid phosphatase) stain using an acid phosphatase leukocyte kit (Sigma). The specimens were then observed under light microscope (Lieca DM 2500, Leica Microsystems, Wetzlar, Germany). The bone area (%) was measured and quantified in the interested area: the distal side of implant surface, using Image Pro Plus 4.5 (Media Cybermetrics, Silver Spring, USA) (Fig. 3). The number of osteocyte with empty lacuna was counted at the same area. TRAP positive cells, indicative of osteoclast, were also counted using IMT i-solution lite version 8.1 program (IMT i-solution Inc., Daejeon, Korea).
To evaluate the effect of bisphosphonate on the expression of DNA at the site of peri-implant bone, microarray analysis was carried out for the rats sacrificed at 6 weeks after starting of injection in both bisphosphonate and control group. The extracted tissue was stored in RNAlater Solutions (Ambion, Austin, TX, USA) and sent to Research and Development Division Korea Institute of Toxicology. Microarray analysis were performed using Rat genome 230 2.0 array (Affymetrix, Santa Clara, CA, USA) and sample labeling, microarray hybridization, washing, and scanning were performed according to the manufacturer's protocol (Affymetrix, Santa Clara, CA, USA).
In order to observe the change in function of osteoblast and angiogenesis activity, the specimens were stained with angiopoietin 1 and osteocalcin antibody using angiopoietin 1 antibody kit (abbiotec LLC, San Diego, CA, USA) and osteocalcin FL-100 kit (Santa cruz biotechnology, Santa Cruz, CA, USA) respectively. Stained specimens were observed under the light microscope.
Mean values and standard deviations of bone area (%), number of empty lacuna and number of tartrate-resistant acid phosphatase (TRAP)-positive cell were calculated. The mean differences were verified with repeated measures analysis of variance and independent t test with a significance level of 5%. All calculations were performed using SPSS ver. 18.0 Program (SPSS ver. 18.0, IBM, NY, USA).
Among the total 30 male Sprague-Dawley rats, one rat from control group (1 week) died during implant placement and two rats from each control group (3 weeks) and bisphosphonate group (6 weeks) also died during recovery period. The other rats appeared to be normal without any significant complication. Microarray analysis was carried out on each sample selected from both control group (6 weeks) and bisphosphonate group (6 weeks). For this reason, the rats sacrificed at 6 weeks after starting of injection in both bisphosphonate and control group were excluded from statistical analysis.
Osteoclast, osteoblast and marrow spaces including mesenchymal cell and blood vessels were identified around implant, indicating that bone remodeling around implant had occurred. Following H&E staining, the bone area was measured within 300 µm area around distal side of implant using light microscope at ×100 magnification and expressed as percentage (%). There was no significant difference between control group and bisphosphonate group in bone area (%). On comparing bone area (%) within control group and bisphosphonate group, no statistically significant difference was found according to duration of administration. (Fig. 4) Similar bone area (48.2%) were observed in each rats sacrificed at 6 weeks after starting of injection from both control group and bisphosphonate group although they are not included for statistical analysis.
Empty lacuna was counted at the same area where bone area measurement (%) was taken. It appeared that empty lacuna in bisphosphonate group increased with the time and,in particular, there was statistically significant difference between control group and bisphosphonate group in the rats sacrificed at 4 weeks after starting of injection (4 week group) (Fig. 5 and Fig. 6). When comparing the number of empty lacuna within each group according to duration of administration, there was no significant difference within control group. However, within bisphosphonate group, the number of empty lacuna in the rats sacrificed at 4 weeks after starting of injection (4 week group) was statistically significantly greater than that of the rats sacrificed at 1 week after starting of injection (1 week group). The rats sacrificed at 6 weeks after starting of injection in bisphosphonate group showed the empty lacuna value of 17.1, which was slightly lower than that of 4 week group but slightly higher than those of 1, 2 and 3 week groups.
The number of osteoclasts using TRAP staining were also counted at the same area and showed no significant difference between control group and bisphosphonate group. On comparing the number of osteoclasts within control group and bisphosphonate group, there was no significant difference according to duration of administration (Fig. 7 and Fig. 8).
Microarray analysis was carried out for the rats sacrificed at 6 weeks after starting of injection in both bisphosphonate and control group. The result of up- and downregulated genes in bisphosphonate group in comparison to that of control group are shown in Table 1.
Immunohistochemical analysis revealed that both bisphosphonate and control group shows pattern of staining along the wall of blood vessel when stained with angiopoietin 1 (Fig. 9). When stained with osteocalcin, which is biomarker of osteoblast, the pattern of staining was also identified at cytoplasm of osteoblast and margin of remodeling cavity in both bisphosphonate and control group (Fig. 10).
Rat model is widely used in many experimental animal model studies. According to previous report,16,17 in rat model, new bone formation and osseointergration was observed within 5 days and 28 days after implantation respectively. It was appeared that, after early osseointegration, bone remodeling phase is also taking place to promote remodeling and reinforcement of bone surrounding implant. In this study, therefore, 4 weeks of healing period (28 days) were allowed to promote bone formation and osseointergration in the maxilla of rats. Bisphosphonates were then injected at the point where bone remodeling phase are thought to be initiated. The volume of bisphosphonates injected (1.0 mg/kg of alendronates) were determined as suggested by Hikita et al.5
From histologic findings, there was no significant difference in bone area (%) between control group and bisphosphonate group. However, the evaluation of effect of bisphosphonate on bone remodeling by only measuring bone area (%) cannot be justified as the osteoclast activity is also reduced by bisphosphonate, hindering the breakdown of bone. Moreover, it was also thought that period of 6 week administration could have been too short to quantify the suppression of bone resorption into bone area (%).
It was previously reported that empty lacuna formed at the early healing period is decomposed by osteoclast, substituted to new osteocyte by osteoblast and gradually disappeared during the normal healing process.12,18 Recently, there was study reporting that empty lacuna was not reduced even after administration of bisphosphonates due to suppressed bone remodeling.19,20 In this present study, the number of empty lacuna was significantly increased in bisphosphonate group with time, especially in rats sacrificed at 4 weeks after initial administration, which is in line with previous study that reported BRONJ like disease.19,20 This result can be interpreted that suppressed function of osteoclast due to administration of bisphosphonates could have led to suppressed bone remodeling and also supported by the result of microarray analysis where up-regulated Sost gene, which inhibits bone formation, was identified. Other possibility is that this can be due to reduced migration of osteoclast, macrophage and monocytes which all act as phagocyte, resulted from suppressed angiogenesis due to administration of bisphosphonates. Increased empty lacuna which appeared in bone remodeling phase could also be related to late failure of bone remodeling phase after implantation. Along the same lines, excessive suppression of bone turnover rate resulting in increased micro-damage accumulation has been emerged as a complication of long-term bisphosphonates administration.21 It was also reported that when microdamage accumulation is increased 2 to 7 folds, bone toughness is reduced by 20% without any effect on bone strength.22 Whyte et al.23 reported the osteopetrosis was observed in patient who received pamidronate for 6 years and spontaneous fracture accompanied by delayed fracture was also observed in 9 patients who received alendronates. In addition, bone formation marker was also extremely reduced in their histologic analysis. Odvina et al.24 reported on excessive suppression of bone turnover following long-term bisphosphonates administration and Visekruna et al.25 also addressed that bisphosphonates could lead to excessive suppression of bone turnover rate, resulting in unfavorable bone fracture. Therefore, taking these studies above as well as the result of the present study into consideration, it can be anticipated that suppression of bone turnover rate induced by a long-term use of bisphosphonates could lead to reduced adaptation of the bone to external stimulus, resulting from inactive empty lacunae around implant and may be one of the most contributing factors for late implant failure. However,its definitive mechanism has not yet been completely clarified.
The result of TRAP staining revealed that there was no significant difference in the number of osteoclast between control group and bisphosphonate group. The clinical efficacy of nitrogen-containing bisphosphonates is thought to be attributed to reduced number of osteoclasts from apoptosis.26-28 However, it was also reported that the number of osteoclast observed in cancellous bone remained unchanged following administration of nitrogen-containing bisphosphonates,29,30 which in turn, means that the mechanism of bisphosphonates can be different from previous hypothesis. There was one study stating that the number of osteoclast increased following alendronates administration in beagle dog.31 Weinstein et al.32 also reported the number of osteoclast increased with the increased dose of alendronates and one third of osteoclasts were found to be giant osteoclasts. When the number of normal osteoclast increases with increase in dose of alendronates, decreased biochemical marker and increased bone density may indicate normal osteoclast is not playing its role properly in absorption of bone. These previous studies showed that nitrogen-containing bisphosphonates could suppress bone resorption without change in number of osteocyte and also suggested that in cancellous bone, the number of osteoclast could be increased instead. In the present study, there was no significant difference in the number of osteoclast between control group and bisphosphonate group.
Although it has been defined that bisphosphonates suppress formation and function of osteoclast through many previous studies,26-28 the effect of bisphosphonate on osteoblast, which always acts in pair with osteoclast during bone remodeling phase, is very scarce. Therefore, in this present study, the concentration of osteocalcin, produced in osteoblast and often used as a marker for bone formation was measured to evaluate the activation and function of osteoblast.
Immunohistochemical analysis revealed that in both control and bisphosphonate groups, the stained osteoclacins were observed in the marginal area where bone remodeling occur as well as in the cytoplasm of osteoblast surrounding medullary cavity. There was no significant difference in the level of osteocalcin staining between control and bisphosphonate groups.33 This result shows that the injection of bisphosphonates has no effect on generation and secretion of osteocalcin. This result is also in agreement with Hirao et al.'s study which evaluated the osteocalcin concentration in blood and the bone mineral density of femur and cervix from 23 menopausal women who received alendronates for one year. In his study, it was reported that the administration of alendronates increased the bone mineral density, but had no effect on the concentration of carboxylated osteocalcin in blood.34
The impairment of angiogenesis, one of the possible mechanisms of BRONJ, was also evaluated in this study by observing the level angiopoietin 1, which is thought to be associated with blood vessel maturation and stability. In contrast to Wood et al.' study35 where marked antiangiogenic properties of bisphosphonates had been reported, in this study, no significant difference was found between control group and bisphosphonate group in the level of angiopoietin 1. Immunohistochemical analysis also revealed that there was no change in the level of angiopoietin 1. It is important to note that from microarray analysis, angiopoietin-like 2, which plays important role in formation of new blood vessel, is down-regulated by half in bisphosphonate group. Therefore, further study may be required to evaluate the effect of bisphosphonate on new blood vessel formation using angiopoietin-like 2 as well as angiopoietin 1.
Although limited sample size and short course of administration could have been limitations of this study, it was confirmed that bisphosphonate administration clearly had an effect on remodeling of peri-implant bone. However, further study may be required to investigate the definitive mechanism of bisphosphonates which suppress differentiation and activation of osteoclast without change in its number and the rationale behind increased number of empty lacunae. Also, in further study, occlusal load applied on implant and surrounding bone tissue, which simulate patients' oral condition should be taken into account since human bone cell is sensitive to the level of mechanical loading with enhanced release of cytokine, nitric oxide (NO), prostaglandin E2 (PGE2) and various growth factors,36,37 which may ultimately lead to different results.
Figures and Tables
Fig. 4
Measurement of bone area (%) in distal side of implant (Cont: control group, Bispho: experimental group)

Fig. 5
Empty lacuna count in H&E stained section (×100). White arrows indicate empty lacunas. (A) Control group of 4-week administration of saline, (B) Bisphosphonate group of 4-week administration of bisphosphonate.
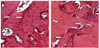
Fig. 6
Empty lacuna cell count: Asterisk (*) indicates statistically significant difference between control group and bisphosphonate group (Cont: control group, Bispho: experimental group).

Fig. 7
Histologic images of TRAP stained sections (×100). White arrows indicate TRAP positive cells. (A) bisphosphonate group/1 week, (B) bisphosphonate group/6 week.
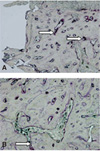
Fig. 9
Immnohistochemical staining of angiopoietin 1 (×200). White arrows indicate stained angiopoietin 1 (Cont: control group, Bispho: experimental group).
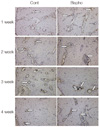
Fig. 10
Immunohistochemical staining of osteocalcin (×200). White arrows indicate cytoplasm of osteoblast and margin of remodeling cavity (Cont: control group, Bispho: experimental group).
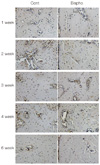
Table 1
Microarray analysis

The result shows prominent disparity of experimental (bisphosphonate) group compared to control group.
*Fgf9: fibroblast growth factor 9, *Klf5: Kruppel-like factor 5, *Sost: sclerostin, *Bmp2: bone morphogenetic protein 2,*Pitx1: paired-like homeodomain transcription factor 1, *IL1β: Interleukin 1beta, *Angptl2: Angiopoietin-like 2.
References
1. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008; 83:1032–1045.
2. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003; 61:1115–1117.
3. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004; 62:527–534.
4. Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009; 67:61–70.
5. Hikita H, Miyazawa K, Tabuchi M, Kimura M, Goto S. Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J Bone Miner Metab. 2009; 27:663–672.
6. Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, Yatani H, Yoneda T. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab. 2010; 28:165–175.
7. Lazarovici TS, Yahalom R, Taicher S, Schwartz-Arad D, Peleg O, Yarom N. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J Oral Maxillofac Surg. 2010; 68:790–796.
8. Grant BT, Amenedo C, Freeman K, Kraut RA. Outcomes of placing dental implants in patients taking oral bisphosphonates: a review of 115 cases. J Oral Maxillofac Surg. 2008; 66:223–230.
9. Smith SY, Recker RR, Hannan M, Müller R, Bauss F. Intermittent intravenous administration of the bisphosphonate ibandronate prevents bone loss and maintains bone strength and quality in ovariectomized cynomolgus monkeys. Bone. 2003; 32:45–55.
10. Allen MR, Kubek DJ, Burr DB. Cancer treatment dosing regimens of zoledronic acid result in near-complete suppression of mandible intracortical bone remodeling in beagle dogs. J Bone Miner Res. 2010; 25:98–105.
11. Ruggiero SL, Drew SJ. Osteonecrosis of the jaws and bisphosphonate therapy. J Dent Res. 2007; 86:1013–1021.
12. Futami T, Fujii N, Ohnishi H, Taguchi N, Kusakari H, Ohshima H, Maeda T. Tissue response to titanium implants in the rat maxilla: ultrastructural and histochemical observations of the bone-titanium interface. J Periodontol. 2000; 71:287–298.
13. Listgarten MA. Soft and hard tissue response to endosseous dental implants. Anat Rec. 1996; 245:410–425.
14. Masuda T, Yliheikkilä PK, Felton DA, Cooper LF. Generalizations regarding the process and phenomenon of osseointegration. Part I. In vivo studies. Int J Oral Maxillofac Implants. 1998; 13:17–29.
15. Pereira MC, Zecchin KG, Campagnoli EB, Jorge J. Ovariectomy delays alveolar wound healing after molar extractions in rats. J Oral Maxillofac Surg. 2007; 65:2248–2253.
16. Fujii N, Kusakari H, Maeda T. A histological study on tissue responses to titanium implantation in rat maxilla: the process of epithelial regeneration and bone reaction. J Periodontol. 1998; 69:485–495.
17. Karimbux NY, Sirakian A, Weber HP, Nishimura I. A new animal model for molecular biological analysis of the implant-tissue interface: spatial expression of type XII collagen mRNA around a titanium oral implant. J Oral Implantol. 1995; 21:107–113. discussion 114-5.
18. Shimizu M, Sasaki T, Ishihara A, Furuya R, Kawawa T. Bone wound healing after maxillary molar extraction in ovariectomized aged rats. J Electron Microsc (Tokyo). 1998; 47:517–526.
19. Bi Y, Gao Y, Ehirchiou D, Cao C, Kikuiri T, Le A, Shi S, Zhang L. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol. 2010; 177:280–290.
20. Kikuiri T, Kim I, Yamaza T, Akiyama K, Zhang Q, Li Y, Chen C, Chen W, Wang S, Le AD, Shi S. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res. 2010; 25:1668–1679.
21. Mashiba T, Turner CH, Hirano T, Forwood MR, Jacob DS, Johnston CC, Burr DB. Effects of high-dose etidronate treatment on microdamage accumulation and biomechanical properties in beagle bone before occurrence of spontaneous fractures. Bone. 2001; 29:271–278.
22. Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001; 28:524–531.
23. Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003; 349:457–463.
24. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005; 90:1294–1301.
25. Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008; 93:2948–2952.
26. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000; 88:2961–2978.
27. Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007; 119:S150–S162.
28. Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998; 19:80–100.
29. Balena R, Toolan BC, Shea M, Markatos A, Myers ER, Lee SC, Opas EE, Seedor JG, Klein H, Frankenfield D. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest. 1993; 92:2577–2586.
30. Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997; 100:1475–1480.
31. Yang Li C, Majeska RJ, Laudier DM, Mann R, Schaffler MB. High-dose risedronate treatment partially preserves cancellous bone mass and microarchitecture during long-term disuse. Bone. 2005; 37:287–295.
32. Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009; 360:53–62.
33. Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001; 16:1575–1582.
34. Hirao M, Hashimoto J, Ando W, Ono T, Yoshikawa H. Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K2 in postmenopausal women. J Bone Miner Metab. 2008; 26:260–264.
35. Wood J, Bonjean K, Ruetz S, Bellahcène A, Devy L, Foidart JM, Castronovo V, Green JR. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002; 302:1055–1061.
36. Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int. 1992; 50:306–313.
37. Klein-Nulend J, Sterck JG, Semeins CM, Lips P, Joldersma M, Baart JA, Burger EH. Donor age and mechanosensitivity of human bone cells. Osteoporos Int. 2002; 13:137–146.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download