Abstract
PURPOSE
This study compared the effect of three intraoral repair systems on the bond strength between composite resin and zirconia core.
MATERIALS AND METHODS
Thirty zirconia specimens were divided into three groups according to the repair method: Group I- CoJet™ Repair System (3M ESPE) [chairside silica coating with 30 µm SiO2 + silanization + adhesive]; Group II- Ceramic Repair System (Ivoclar Vivadent) [etching with 37% phosphoric acid + Zirconia primer + adhesive]; Group III- Signum Zirconia Bond (Heraus) [Signum Zirconia Bond I + Signum Zirconia Bond II]. Composite resin was polymerized on each conditioned specimen. The shear bond strength was tested using a universal testing machine, and fracture sites were examined with FE-SEM. Surface morphology and wettability after surface treatments were examined additionally. The data of bond strengths were statistically analyzed with one-way ANOVA and Tamhane post hoc test (α=.05).
RESULTS
Increased surface roughness and the highest wettability value were observed in the CoJet sand treated specimens. The specimens treated with 37% phosphoric acid and Signum Zirconia Bond I did not show any improvement of surface irregularity, and the lowest wettability value were found in 37% phosphoric acid treated specimens. There was no significant difference in the bond strengths between Group I (7.80 ± 0.76 MPa) and III (8.98 ± 1.39 MPa). Group II (3.21 ± 0.78 MPa) showed a significant difference from other groups (P<.05).
Yttrium oxide partially stabilized tetragonal zirconia polycrystal (Y-TZP), introduced to the prosthodontic field in the early 1990s as an all ceramic core material,1 has favorable esthetic characteristics, superior mechanical properties and biocompatibility.2-6 Due to its mechanical properties such as high strength toughness, it was difficult to handle with manual laboratory method. Recently, the development of dental CAD/CAM technology allows the design and fabrication of zirconia based restoration for all ceramic restoration to be faster and have better practical use2,4,7 In addition, according to the development of various zirconia blocks with diverse colors and strengths, the range of clinical use is on the expansion gradually.
The full zirconia crown has less silica content than other glass ceramic systems and the resultant low translucency makes it difficult to express natural colors. Therefore, especially for the anterior restorations, zirconia copings for crowns or multi-unit frameworks still require application of veneering porcelain to achieve suitable esthetics.5 Although such a 2 layer structure could make diverse colors more efficient, weak veneering porcelain over a strong supporting core could be delaminated or fractured.8,9 Chipping of veneering porcelain accompanied with the exposure of the zirconia coping during the use within the oral cavity may be considered as a type of bonding failures between the zirconia core and the veneer.10,11
Such a failure is caused by surface treatment of the core for mechanical retention, residual stresses generated by mismatch in coefficient of thermal expansion (CTE), development of flaws and structural defects at core-veneer interface, and wetting properties and volumetric shrinkage of the veneer.3-5
Clinically because of such variable factors, chipping of veneer is the most frequently reported complication of zirconia based ceramics.9,12 Replacement of a failed restoration is ideal for the fracture, but is not necessarily the most practical solution due to replacement cost, additional trauma to the tooth and other limited factors in patients. In addition, for cases with a small fracture area that does not cause esthetic or functional problems, intraoral repair techniques with composite resin may be more effective methods.8
Various intraoral repair systems have been developed and used to optimize the fractured surface and to improve their bond strength with composite resin. However, most of studies on the clinical usefulness of intraoral repair systems are limited to the bond strength between composite resins to fractured porcelain or exposed metal surfaces in porcelain fused metal crown. But, the studies on the bond strength of composite resin to zirconia by the application of intraoral repair systems are insufficient. Nowadays, some manufacturing companies are providing intraoral repair systems for zirconia surface treatment.
Therefore, the purpose of this in vitro study was to evaluate the effect of 3 different intraoral repair systems on the shear bond strength of composite resin to zirconia.
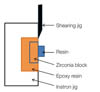
Thirty zirconia specimens (10 mm × 10 mm × 2 mm) were produced by a CAD/CAM system (Zirkonzahn, Italy) using prefabricated blocks of zirconia (Prettau Zircon; Zirkonzahn, Italy) and then sintered according to the manufacturer's instructions. Zirconia specimens were embedded in liquid unsaturated polyester (EPOVIA® RF-9000, Cray valley Inc., Korea) (Fig. 1). The surface of all the specimens were grounded with 100, 600, 1200 grit silicon carbide abrasive discs under running water on a polishing machine (Buehler Metaserv, Buehler, Germany), then ultrasonically cleaned for 3 minutes in deionized water and then wiped with 95 vol.% ethanol. Clinically, when the fracture site is repaired with composite resin, surface grinding with diamond bur is a commonly used method for roughening the surfaces to improve mechanical bonding and remove the contamination area. To simulate this clinical condition, all specimens were ground using a fine-grit diamond rotary cutting instrument (Red stripe round-end straight diamond bur, 30 µm grit size; Komet®, Germany) in a high-speed handpiece with water irrigation for 10 seconds.
Specimens were randomly divided into three groups, each containing 10 specimens, for the following different intraoral repair systems.
Group I (n=10): CoJet™ Intraoral Adhesive Repair System (3M ESPE, Seefeld, Germany). The specimens were airborne-particle, abraded using a chairside abrader (Ultra-Blaster, Ultradent Products Inc., USA) for 15 seconds with 30 µm silica-modified Al2O3 particles (CoJet™) with 3.0 bar pressure from 10 mm distance. After abrasion, the specimens were thoroughly rinsed with a water spray for 30 seconds to clean the surface residual sand particles, and then dried with oil-free air. Specimens were treated with prehydrolyzed silane-based primer (Rely X™) and then dried with oil-free air for 30 seconds. A bonding agent (Adper™ Single Bond 2 Adhesive) was then applied a thin layer with a disposable brush and light cured for 10 seconds.
Group II (n=10): Ceramic Repair System (Ivoclar Vivadent, Schaan, Liechtenstein). The specimens were etched with 37% phosphoric acid gel (Total Etch) for 15 seconds, rinsed with water spray for 30 seconds, and then air dried until a frosty white appearance was observed. Zirconia primer (Metal/zirconia primer) was applied on the zirconia surface for 180 seconds, then air dried gently, and an adhesive bonding agent (Heliobond)was applied a thin layer and light cured for 20 seconds.
Group III (n=10): Signum Zirconia Bond (Heraeus Kulser, Milan, Italy). The specimens were treated with a bifunctional methacrylate based component containing acetone and MDP (Signum Zirconia Bond I and dried with oilfree air. A bonding agent containing methyl methacrylate and diphenyl (2,4,6-trimethylbenzoyl) phosphinoxide (Signum Zirconia Bond II) was then applied to the zirconia surface with a disposable brush and light-cured for 40 seconds.
After surface treatment according to manufacturer's manual of each intraoral repair system, plastic cylinder (6 mm diameter, 4 mm length) was placed on the center of the specimens, and composite resin (Z350, 3M ESPE, USA) was placed condensed into the cylinder and incrementally filled up, while each layer was light-polymerized for 40 seconds at a distance of 1 mm using a light-polymerizing unit (Astralis 3, Ivoclar Vivadent, Liechtenstein) with an output power of 600 mW/cm2. After removing the plastic cylinder, an additional 40 seconds of polymerization was performed. Specimens were stored in distilled water at 37℃ for 72 hours before shear bond testing. The materials used, compositions and manufacturer's details are presented in Table 1.
Each specimen was then mounted in the universal testing machine (AGS-1000D, Shimadzu, Japan), equipped with a load which was applied to the adhesive interface until failure occurred (cross-head speed of 1.0 mm/min) (Fig. 2). The ultimate load to failure was recorded in Newton (N). The average bond strength (MPa) was calculated by dividing the maximum ultimate load (N) to failure by the area of the composite resin (mm2).
After shear bond testing, debonded specimen surfaces were examined by FE-SEM to assess the failure mode. The mode of failure was classified as either cohesive failure in the composite resin, or interfacial adhesive failure at the zirconia-resin interface.13 Results of the shear bond test were statistically analyzed with one-way ANOVA (SPSS 15.0, SPSS Inc., Chicago, IL, USA), followed by a Tamhane post hoc test (α=.05).
To evaluate the effect of surface treatment methods, five additional zirconia specimens were prepared. The surface morphologies, roughness patterns and wettability were evaluated on the polished, diamond bur treated, blasted with CoJet sand, 37% phosphoric acid treated and Zirconia Bond I applied surfaces. All specimens were examined under a field emission scanning electron microscope (S-4800, Hitachi, Japan) at 15 kV. The FE-SEM images were developed at a magnification of ×1000 for visual inspection. Also, the surface roughness was examined under 3 dimensional optical microscope (Nikon LV150L, Japan). Wettability value was evaluated using a water contact angle goniometer (Kruss DAS100, Germany) in sessile drop mode with 5 µL drops.
The mean shear bond strength values and differences between groups are shown in Table 2. The shear bond strength of Group I (7.80 ± 0.76 MPa) and Group III (8.98 ± 1.39 MPa) were found to be significantly higher than the Group II (3.21 ± 0.78 MPa). There was no significant difference between Group I and Group III.
The surface morphologies were analyzed, after the polishing of zirconia specimen, after the treatment with a diamond bur, after the treatment with CoJet sand in Group I, after the treatment with 37% phosphoric acid in Group II, and after the treatment of Zirconia Bond I in Group III. FE-SEM and 3D-OM images of treated zirconia surfaces were shown in Fig. 3.
When compared with polished zirconia specimens, the surface irregularities of the specimens of all groups were increased. The polished surfaces generally showed smoothness in spite of small grooves due to polishing process. 30 µm diamond bur treated surface led to rough surfaces and defects according to the moving direction of the bur. On the surface of the specimens treated with CoJet sand in Group I, the surface irregularity was distinctly increased and small nodules were observed. The surface pattern of the Group II treated with 37% phosphoric acid was similar to the pattern after the treatment with a diamond bur, but the surface remnants in the bur treatment group were removed due to the cleaning effects of phosphoric acid. The surface of the Group III after the use of Zirconia Bond I showed smootherpatterns than diamond bur treated surfaces by covering of surface irregularities.
During the specimen preparation process of each group, contact angle value of all of specimens was measured as an indication of surface wettability and surface bonding strength. Increasing surface roughness has previously been shown to improve the wettability and reduce the contact angle value. Fig. 4 shows the contact angle values of polished surface, diamond bur treated surface, silica coated surface with CoJet sand in Group I, 37% phosphoric acid etched surface in Group II, and Zirconia Bond I applied surface in Group III. The contact angles after surface treatment of the all groups were decreased in comparison with polished surface (68.96°) and diamond bur treated only (53.55°). The value of contact angle decreased significantly in the order of 37% phosphoric acid etched surface in Group II (52.24°) > Zirconia Bond I applied surface in Group III (38.29°) > silica coated surface with CoJet sand in Group I (17.34°). Particularly, the contact angle value after CoJet sand treatment in Group I was measured to be noticeably lowers than those of other groups; thus excellent surface wettability was shown (Fig. 4).
The modes of failure after the shear bond test of all specimens are shown in Fig. 5, Fig. 6 and Fig. 7. Some specimens of Group I and III with strong bond strength showed a combination of adhesive and cohesive failure, with bonding agent partly remaining on the zirconia surface (Fig. 5 and Fig. 7). However, fracture site of Group II specimens showed adhesive failure mode (Fig. 6).
Layering techniques that build-up porcelain onto the zirconia core are methods that could compensate the limitation of the color of zirconia blocks, and they have been used for anterior restoration to achieve suitable esthetics. A weak bond between the veneering porcelain and zirconia core can result in fracture or delamination of the veneer itself.4 According to studies by Sailer et al.,14,15 the clinical failure rate caused by chipping of the veneering porcelain was reported to be 13% after three years and 15.2% after five years.
The delamination of veneering porcelain from zirconia core can be caused by excessive shear stress which is induced by continuous occlusal interference or excessive tensile stress which is accumulated in the veneering porcelain during the porcelain build up and firing. Such tensile stress in the veneer generated by mismatch in coefficients of thermal expansion (CTE).4 In the metal-ceramic restoration, for the close bonding of metals and porcelain, the CTE of metals is higher than that of veneering porcelain by approximately 0.5 × 10-6/℃.16 Particularly, if the precious metals is used as a core, the difference of CTE may be compensated by the creep deformation caused by metal heat.17,18 However, the strength of zirconia core is very high and such creep deformation is absent. Therefore, the risk for developing destructive stress in the upper veneering porcelain is higher. In the zirconia restorations, the CTE of porcelains that are developed as zirconia ceramics currently is 9-10 × 10-6/K. In comparison with the CTE of feldspathic porcelain (12-15 × 10-6/K) that has been used in the general metal-porcelain restoration, the CTE of zirconia ceramics is comparable to that of partially stabilized zirconia (9-11 × 10-6/K) and accelerates the bonding to zirconia.5
The fractures of the veneering porcelain results in loss of financial and time frompatients. Particularly, in patients whose systemic condition renders from remaking the restoration, intraoral repairing methods may be more realistic treatments. Especially, with the development of zirconia restorations, some manufacturing companies introduced intraoral repair systems for zirconia restorations. As a general porcelain repair system is based on the bonding strength between porcelain and composite resin, in cases with the exposed zirconia core, the prognosis of the intraoral repair would be determined by the increase of the bonding strength of zirconia surface and composite resin. Generally, the methods that improve the bonding strength of porcelain to resin could be divided to mechanical bonding and chemical bonding.12,17 Methods that render roughness by using HF or phosphoric acid, sandblasting, or diamond burs may be considered as mechanical methods. Methods that use silane or primers may be considered to be chemical ones. It was confirmed that such methods improved the bonding strength of porcelains and not the zirconia. Zirconia is minimally influenced by general methods that improve surface roughness.7,19-22 Most studies that examined the effect of phosphoric acid or HF have reported that the effect of acid on zirconia is meager.3,5,19
Under clinical situation, restorative luting surfaces can be contaminated by saliva, blood or silicon et al. Saliva contamination is frequently the main reason for reduced resin bond strength. Generally, the application of phosphoric acid on zirconia surface is recommended to facilitate the bonding of composite resin by removing the contamination rather than increasing the surface roughness. Such effects could also be obtained by using acetone or alcohol.2,19 In our study, the specimens treated with 37% phosphoric acid in Group II and treatment with Zirconia Bond I containing acetone components in Group III showed clearer surface than the surface treated with diamond burs, which may be considered to be the effect of the removal of surface contamination by phosphoric acid or acetone.23
Diverse effects of sandblasting with airborne particles on zirconia surface have been reported. Sandblasting has been identified as an effective method in achieving a stable, durable bond for zirconia based ceramics.7,19 However, excessive air pressure delivered during blasting can induce the phase change of zirconia or microcracks reducing the mechanical property of zirconia.5,24,25 In addition, if zirconia is used as the core, sufficient roughness could not be formed by sandblasting.8,26,27
For high strength ceramics such as zirconia or alumina, silane coupling agents are not effective due to their low silica contents. Since the silica content of common feldspathic porcelain is 50-60 wt%, and the silica content of zirconia is lower than 1 wt%, alumina and zirconia ceramic materials do not react effectively with silane coupling agents.8 Matinlinna et al.28 have reported that 3-methacryloxypropyltrimethoxy silane (3MPTS) is a well-known silane that improves the bonding strength to resin by reacting efficiently on the ceramic surface containing silica, but does not provide effective bonding strength when used alone on zirconia. In our study, similarly, it was observed that zirconia primers and silane used in Group II contained such 3MPTS components, but did not provide effective bonding strength to zirconia of which silica content is low.
CoJet™ System used in our study is composed of blasted silica modified Al2O3 particles, which promotes surface roughness and silica coat for resin bonding via silane agents (Rely X).8,19 In our study, it was observed that after the treatment of zirconia surface with CoJet™ System, microroughness and surface wettability were improved greatly, and the shear bond strength with composite resin was enhanced. Thus, it is considered that increased surface area due to silica coating and the additional chemical bonding with silane agents improved the bonding strength. Other studies on the chemical bonding of zirconia have reported that the use of the phosphate ester monomer 10-MDP provided stable bondings.13,29,30 The reason is that the phosphate ester group of MDP binds directly to the zirconia oxide layer with high bonding strength. The Signum Zirconia Bond in our study also contains such 10-MDP, and provides excellent bonding strength to increase the chemical bonding strength of zirconia surface; these findings conform to previous studies.
For the evaluation of the bond strength between resin and dental porcelains, shear bond test, tensile test, microtensile test, and other diverse tests have been applied.31 Of them, the advantages of shear bond test applied in our study are that specimens could be prepared easily, clear test protocols are provided, and test results could be analyzed rapidly. On the other hand, shortcomings of the shear bond strength test are that deviations occur widely depending on the quality of specimen.32 Hence, it has been reported that in experiments which assessed the shear bond strength between porcelain and composite resin, cohesive failures in which fractures occured in porcelain rather than in the resin bond area were observed, and it was difficult to measure the absolute value of bond strength.19,33 However, because zirconia specimens used in our study were high-strength and sintered, fractures occurred in the interfacial surface and thus more reliable values of bond strength were revealed.
Intraoral prostheses are exposed to the continuous changes of humidity and temperature. The limitation of in vitro experiments is that they could not represent intraoral environment. There are some methods that endow such environment by long term storage and thermocycling in water. Some reports said that such treatments induces the weakening of bond strength13,22, but does not exert special effects on statistical analysis.34
In our study, only a short storage period was provided by storing specimens at 37℃ for 72 hours. As further studies, the change of bond strength after long-term storage as well as thermal cycling treatments is required. And also, the comparison of the bond strength after repeated fatigue loadings is required additionally.
Within the limitations of this in vitro study, the use of intraoral silica coating system (CoJet™system) and the application of Signum Zirconia Bond enhances the shear bond strength between composite resin and zirconia. Intraoral silica coating system used in this study represents a feasible method for improving zirconia surface roughness and wettability. However, the presence of MDP and methyl methacrylate in the Signum Zirconia Bond may increase the bond strength by the formation of chemical bonds between composite resin and zirconia.
Figures and Tables
 | Fig. 3FE-SEM images (magnification ×1000, left) and 3D-OM images (right) of the zirconia surface after different surface treatments. (A) Polished, (B) diamond bur treated, (C) Group I, CoJet sand treated, (D) Group II, 37% phosphoric acid etching, (E) Group III, Zirconia Bond I applied. |
 | Fig. 4Contact angle values of zirconia surface after different surface treatments. (A) Polished, (B) Diamond bur treated, (C) Group I, CoJet sand treated, (D) Group II, 37% phosphoric acid etching, (E) Group III, Zirconia Bond I applied. |
 | Fig. 5FE-SEM images of the interfacial patterns observed in Group I. (A) Fractured surface after the shear test, zirconia side (magnification ×40), (B) High magnification of arrow area in A (magnification ×1000). Retained bonding agent remnants were detected. |
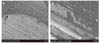 | Fig. 6FE-SEM images of the interfacial patterns observed in Group II. (A) Fractured surface after the shear test, zirconia side (magnification ×50), (B) High magnification of arrow area in A (magnification ×1000). No residue of bonding agent and resin was detectable on the surface. |
References
1. Choi BK, Han JS, Yang JH, Lee JB, Kim SH. Shear bond strength of veneering porcelain to zirconia and metal cores. J Adv Prosthodont. 2009; 1:129–135.
2. Jang GW, Kim HS, Choe HC, Son MK. Fracture strength and mechanism of dental ceramic crown with zirconia thickness. Procedia Eng. 2011; 10:1556–1560.
3. Ural C, Külünk T, Külünk S, Kurt M, Baba S. Determination of resin bond strength to zirconia ceramic surface using different primers. Acta Odontol Scand. 2011; 69:48–53.
4. Chaiyabutr Y, McGowan S, Phillips KM, Kois JC, Giordano RA. The effect of hydrofluoric acid surface treatment and bond strength of a zirconia veneering ceramic. J Prosthet Dent. 2008; 100:194–202.
5. Thompson JY, Stoner BR, Piascik JR, Smith R. Adhesion/cementation to zirconia and other non-silicate ceramics: where are we now? Dent Mater. 2011; 27:71–82.
6. Triwatana P, Nagaviroj N, Tulapornchai C. Clinical performance and failures of zirconia-based fixed partial dentures: a review literature. J Adv Prosthodont. 2012; 4:76–83.
7. Ural Ç, Külünk T, Külünk Ş, Kurt M. The effect of laser treatment on bonding between zirconia ceramic surface and resin cement. Acta Odontol Scand. 2010; 68:354–359.
8. Kim BK, Bae HE, Shim JS, Lee KW. The influence of ceramic surface treatments on the tensile bond strength of composite resin to all-ceramic coping materials. J Prosthet Dent. 2005; 94:357–362.
9. Cho HY, Won HY, Choe HC, Son MK. Fracture characteristics of dental ceramic crown according to zirconia coping design. Procedia Eng. 2011; 10:1561–1566.
10. Hatta M, Shinya A, Yokoyama D, Gomi H, Vallittu PK, Shinya A. The effect of surface treatment on bond strength of layering porcelain and hybrid composite bonded to zirconium dioxide ceramics. J Prosthodont Res. 2011; 55:146–153.
11. Choi YS, Kim SH, Lee JB, Han JS, Yeo IS. In vitro evaluation of fracture strength of zirconia restoration veneered with various ceramic materials. J Adv Prosthodont. 2012; 4:162–169.
12. Casucci A, Monticelli F, Goracci C, Mazzitelli C, Cantoro A, Papacchini F, Ferrari M. Effect of surface pre-treatments on the zirconia ceramic-resin cement microtensile bond strength. Dent Mater. 2011; 27:1024–1030.
13. Aboushelib MN, Kleverlaan CJ, Feilzer AJ. Selective infiltration-etching technique for a strong and durable bond of resin cements to zirconia-based materials. J Prosthet Dent. 2007; 98:379–388.
14. Sailer I, Fehér A, Filser F, Lüthy H, Gauckler LJ, Schärer P, Franz Hämmerle CH. Prospective clinical study of zirconia posterior fixed partial dentures: 3-year follow-up. Quintessence Int. 2006; 37:685–693.
15. Sailer I, Fehér A, Filser F, Gauckler LJ, Lüthy H, Hämmerle CH. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int J Prosthodont. 2007; 20:383–388.
16. Wataha JC. Alloys for prosthodontic restorations. J Prosthet Dent. 2002; 87:351–363.
17. Fischer J, Baltzer N, Fleetwood PW. Thermal creep analysis of noble metal alloys for the ceramic-fused-to-metal technique. J Biomed Mater Res. 1999; 48:258–264.
18. Anusavice KJ, Carroll JE. Effect of incompatibility stress on the fit of metal-ceramic crowns. J Dent Res. 1987; 66:1341–1345.
19. Della Bona A, Borba M, Benetti P, Cecchetti D. Effect of surface treatments on the bond strength of a zirconia-reinforced ceramic to composite resin. Braz Oral Res. 2007; 21:10–15.
20. Park JS, Kim HS, Kim HSL, Song MK, Choe HC. Interfacial Bonding and Fracture Phenomena between Porcelain and Metal Coping. Procedia Eng. 2011; 10:1567–1572.
21. Son MK, Choe HC. Evaluation of Interfacial Bonding Strength between Laser Textured Metal Coping and Porcelain. Procedia Eng. 2011; 10:2268–2291.
22. Qeblawi DM, Muñoz CA, Brewer JD, Monaco EA Jr. The effect of zirconia surface treatment on flexural strength and shear bond strength to a resin cement. J Prosthet Dent. 2010; 103:210–220.
23. Yang B, Wolfart S, Scharnberg M, Ludwig K, Adelung R, Kern M. Influence of contamination on zirconia ceramic bonding. J Dent Res. 2007; 86:749–753.
24. Zhang Y, Lawn BR, Malament KA, Van Thompson P, Rekow ED. Damage accumulation and fatigue life of particle-abraded ceramics. Int J Prosthodont. 2006; 19:442–448.
25. Zhang Y, Lawn BR, Rekow ED, Thompson VP. Effect of sandblasting on the long-term performance of dental ceramics. J Biomed Mater Res B Appl Biomater. 2004; 71:381–386.
26. Gökçe B, Ozpinar B, Dündar M, Cömlekoglu E, Sen BH, Güngör MA. Bond strengths of all-ceramics: acid vs laser etching. Oper Dent. 2007; 32:173–178.
27. Fischer J, Grohmann P, Stawarczyk B. Effect of zirconia surface treatments on the shear strength of zirconia/veneering ceramic composites. Dent Mater J. 2008; 27:448–454.
28. Matinlinna JP, Lassila LV, Vallittu PK. The effect of a novel silane blend system on resin bond strength to silica-coated Ti substrate. J Dent. 2006; 34:436–443.
29. Blatz MB, Sadan A, Martin J, Lang B. In vitro evaluation of shear bond strengths of resin to densely-sintered high-purity zirconium-oxide ceramic after long-term storage and thermal cycling. J Prosthet Dent. 2004; 91:356–362.
30. Wegner SM, Kern M. Long-term resin bond strength to zirconia ceramic. J Adhes Dent. 2000; 2:139–147.
31. Ersu B, Yuzugullu B, Ruya Yazici A, Canay S. Surface roughness and bond strengths of glass-infiltrated alumina-ceramics prepared using various surface treatments. J Dent. 2009; 37:848–856.
32. Valandro LF, Ozcan M, Amaral R, Vanderlei A, Bottino MA. Effect of testing methods on the bond strength of resin to zirconia-alumina ceramic: microtensile versus shear test. Dent Mater J. 2008; 27:849–855.
33. Chadwick RG, Mason AG, Sharp W. Attempted evaluation of three porcelain repair systems-what are we really testing? J Oral Rehabil. 1998; 25:610–615.
34. Lindgren J, Smeds J, Sjögren G. Effect of surface treatments and aging in water on bond strength to zirconia. Oper Dent. 2008; 33:675–681.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download