Abstract
PURPOSE
The purpose of this study was to assess the difference in efficacy between calcium metaphosphate (CMP)-coated implant fixtures and conventional resorbable blasted media (RBM) processed implant fixtures.
MATERIALS AND METHODS
This study targeted 50 implants from 44 patients who visited Dankook University Dental Hospital. Implantations were done separately for RBM treated and CMP-coated implants, although their design was the same. Calcium metaphosphate has a quicker biodegradation process through hydrolysis compared to other phosphate calcium groups. For the first year of the implantation, the resorption volume of marginal bone analyzed via radiography and perio-test value were measured, under the check plan. Their analyses were composed of a non-inferiority trials test. A 95% level of reliability was used.
Since Machined surface titanium implants were introduced by Brånemark, they have been used not only for fully edentulous patients but also for those who are partially edentulous for functional restoration.1,2 More types of implants have been developed including subperiosteal implants and blade-vent implants, although root-type osseointegrated implants are chiefly used at present. Albrektsson defined osseointegration as: implant and bone tissue making direct contact without connective tissue and movement when observed under an optic microscope.3 He also found that implant material, design, surface, bone condition, surgical method, and loading condition are crucial factors in achieving osseointegration. In particular, implant material, design, and surface are believed to be related to implant quality; active studies on implant surface and design aspects have been ongoing. A major change in implant surface is the shift from smooth and glossy surface to rough. Carlsson et al.4 found that implants with irregular surfaces form stronger mechanical adhesion with the surrounding bone tissue than those with glossy surface and the mechanical adhesion at the early stage can result in a stable osseointegration by preventing a fine movement. As methods of processing a glossy surface into a rough one, coating, polishing, blasting, etching, and anodic oxidation are used; some of these are under research.5 Hydroxyapatite is akin to an ingredient of bone tissue; it is known for its affinity with the implantbone interface.6 In terms of the coating method with hydroxyapatite onto implants, plasma spraying, the most common method, dipping, electrodeposition, and pulsed-laser deposition are used.
The surface of hydroxyapatite-coated implants has generally been formed by spraying hydroxyapatite powder at high pressure onto a titanium surface to create a hydroxyapatite layer.7 Based on the results of a long-term animal test, however, the hydroxyapatite-coated layer does not biodegrade even after a considerable period of time, retaining its adhesion with bone tissue instead. Nonetheless, concerns over the detachment of the interface between the metal implant and the hydroxyapatite-coated layer have been reported. Some studies seeking to resolve this problem have been underway.8 If quickly degradable substances such as tricalcium phosphate (TCP) or calcium metaphosphate (CMP) are coated onto implants, prompt adhesion with bone tissue is realized, and biodegradation occurs along with the growth of the bone tissue in a relatively short time; thus inducing direct adhesion with implant fixtures. CMP ([Ca(PO3)2]n) has a polymer structure consisting of phosphate group chains and is involved in a quicker biodegradation process through hydrolysis compared to other phosphate calcium groups.9,10 Those who conformed to the following criteria were excluded: In vitro study about biocompatibility of CMP with human bone marrow stromal cells, CMP disks produce better results than HA disks.11 The most commonly used CMP coating procedure is a dipspin coating technique.12 Implants that were coated this way showed increased or constant ISQ values during the initial healing period in animal Experiment.13
Accordingly, this study compared the CMP-coated fixtures and powdered hydroxyapatite-blasting RBM (Resorbable Blast Media) fixtures placed in the oral cavities of patients.
The project for this study was given an official recognition by the Ethics Committee of Dankook University (IRB no. 2004005), being also in compliance with the helsinki declaration. Among those who visited Dankook Univ. Dental Hospital for implant treatment, the 50 implants from 44 patients who needed the placement of one or two implants (next to each other) were targeted. Those who conformed to the following criteria were excluded:
Criteria for test target assignment14,15 is as follows. Patients who lost their maxillary and mandibular teeth and wish to get implant treatment instead of having their adjacent teeth cut and wearing a removable prosthesis agreed to participate in the clinical test, signed a tester agreement, and met the following criteria (cases with deficient antagonist teeth on the implant site were excluded; note, however, that patients who had planned the prosthetic restoration of antagonist teeth were included in the test): patients whose jawbone has stopped growing, good condition of oral cavity and those with clear desire for implant treatment.
The following cases did not fit this clinical test, and they were considered absolute taboo cases for implant treatment: pregnant women, patients with recent episode of myocardial infarction, patients with internal ailment that cannot be controlled, patients with hemorrhagic ailment, patients who have mental illness, patients who are not cooperative, patients who are allergic to implant material, patients in a growth phase and patients who are uncertain about implant treatment.
Random method for selection and the determination of a comparison group is as follws. The selection of non-submerged RBM dental implant SSII or CMP-coated implant SSII Type was determined randomly according to specific allocation chart. A research nurse managed the chart; once the final selection of testees was made, grouping was done based on the chart. A testee identification code was then marked in the chart. The test group and a control group were randomly determined through stratified block randomization. For the selection, the test group was selected in a number that was twice that of the control group. With regard to the intermaxillary ratio, the maxilla was selected for at least 30% of the total; for the anterior versus posterior, the anterior was selected for at least 15%.
For the basic shape of implants, SS II systems (Osstem, Seoul, Korea) were used. For the control group, the RBM surface blasted with hydroxyapatite [Ca10(PO4)6(OH)2] powder was used to increase the surface roughness. Similar to the control group, CMP-coated products were used onto the RBM-processed surface for the test group. The CMP solutions were mixed Calcium nitrate tetra hydrate [Ca(NO3)24H2O] and triethyl phosphite [P(OC2H5)3] in methanol. The resultant CMP sol-gel was Ca/P molar ratio of 0.5. The RBM surface implants were dipped in CMP solution and sintered at 630℃ for CMP-coated products.
Implant placement was carried out according to the recommendations of the manufacturer. Based on the planned procedure, routine checkups and prosthetic creation were then done. The procedure is described below (Table 1).
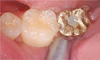
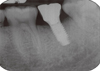
One year after the placement of fixtures, Periotest (Siemens AG, Bensheim, Germany) was used. The Periotest value (PTV) was measured at the prosthetic level (Fig. 1). To reduce the measurement error, measurement was repeated 3 times until the same value was measured. For the radiographic scan, Kodak insight film (Kodak Co, California, USA) placed perpendicularly from the implant fixtures was used (Fig. 2). Afterward, using an Epson expression 1600 pro scanner (Seiko Epson Co., Nagano, Japan), scanning was done at 1,200 dpi resolution. A 1,024 × 765-pixel Syncmaster 155MP monitor (Samsung Electronics, Suwon, Korea) was used for all measurements by one person. The measurements for bone absorption were based on the first screw helix of implant fixtures and were revised with the pitch of the fixtures as referenced.
For the evaluation of the success rate of this clinical test, all implants placed in the testees were targeted. One year after the implant placement, observations were made on abnormal reactions including implant looseness, discomfort, radiographic image of area around the implant fixtures, and absorption of marginal bone. Occlusal assessments were then made, and patients received instruction on oral hygiene.
Major evaluation factor used to determine the success or failure of dental implants during the first full year after the implant surgery is as follows. Functioning implants are classified into the following 4 types:16 "Unaccounted" indicates those implants that failed the observations for unknown reason. "Failure" is implants that were removed for some reason. "Survival" is group except 1 and 2. "Success" is group conforming to the following success criteria. Success criteria for dental (osseointegrated) implants is as follows.17,18 First, there is no consistent or non-reciprocal discomfort, pain, or abnormal sensations. Secondly, there is no recurrence of peri-implant inflammation accompanying abscess. Thirdly, there is no mobility. Lastly, there should be no peri-implant radiographic lesion. If any of the above criteria is not met, the group is considered a failure.
The secondary factors of this clinical test were used as reference to determine the success rate through diagnostic parameters that verify the factors affecting the result (success or failure) of implant treatment. It should be less than 1 mm during the first full year after implant placement and less than 0.2 mm a year thereafter.19-22 The degree of osteolysis is evaluated based on the periapical radiography. The group is considered a failure if it does not conform to these criteria. This case is included in the survival rate but not in the success rate. The evaluation of bone quality is based on both the sensation during bone drilling for implant surgery and panoramic radiography.23
All implants should have no mobility. Solid bonding is tantamount to zero clinical mobility when tested vertically or horizontally below 500 g; this is similar to the manner of assessing that of teeth. The absence of clinically observable mobility does not mean zero actual mobility. A natural tooth with mobility actually has 56-73 µm horizontal mobility. A healthy implant moves less than 75 µm, and its clinical mobility is 0.24 As a non-destructive test that is clinically available for the examination of osseointegration and stability of implants, Perio-test measurement was included. The existence of implant mobility means failure of osseointegration; since a PTV value is not absolute, however, assessment should be done in connection with the clinical and radiographic index. There should be no suppuration around the implants.25
Occlusion evaluation is as follows26,27 : From a long-term perspective, occlusal stability can improve the prognosis of implant treatment by preventing prosthetic overload. For adequate occlusal adjustment, tension and deformation occurring from the bone-implant contact point should be within the range of biological stimulation. However implants have no periapical membrane. Thus, patients need to be advised on light tapping and strong tooth clenching during occlusal adjustment. Adjacent teeth and early occlusal contact should be eliminated completely. Using Shimstock (8 µm thick), evaluate the fit or misfit. When light clenching is applied using shim-stock, 1-2 sheets of Shimstock slip out of the prosthetic implants. When biting is applied onto two overlapped sheets, adjust the occlusion so that it sticks. The thickness of 2 sheets is 16 µm, and that of 3 sheets, 24 µm. Clenching with 3 sheets is required to prevent excessive occlusal gap. In other words, light clenching makes shim-stock slip away freely; heavy contact makes it slip away even in cases of slight resistance. When light tapping is applied after Accu-Film II (Red/Black-two-sided) articulating paper is inserted, the occlusal contact on the implant prosthesis needs to be reduced if the same extent of occlusal contact on the implant and natural teeth is detected. Again, when 1 sheet of Accu-Film II is clenched with light occlusal contact, there should be no contact on the implant. During the vertical movements of the mandibular jaw, if natural teeth with periodontal ligament do not move down vertically, a fine occlusal gap measuring approximately 20 µm (Accu-Film II thickness) between the implant prosthesis and the antagonist tooth is required to prevent overload on the implant. This is because it protects from 8-28 µm vertical tooth movement that may occur during strong mastication. When stronger occlusal contact is formed, light occlusal contact should be achieved onto the implant prosthesis. In other words, the measurement should ensure that light occlusal contact indentation is formed compared to adjacent natural teeth.
For all measurements, Windows SPSS V. 12.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical processing. With regard to the comparison of the mean values of the absorption level of marginal boneboth in the control group and the test group for each period and PTV one year after the placement of fixtures, the independent t-test was used to verify the significance. For the time-based difference in the absorption of marginal bone, the significance was verified through the paired t-test. In particular, for the time-based difference comparison, the maxillae and the mandibles were divided according to Visit 8. A 95% level of reliability was used.
One year after the placement of fixtures, the mean PTV of the test group was found to be higher than that of the control group. In the independent t-test result, however, no statistically significant difference was found (Table 2).
The absorption levels of marginal bone in the test group for each period were lower than those of the control group; in the independent t-test result, however, no statistically significant difference was found in all periods (Table 3).
For the period-based difference in the mean absorption levels of marginal bone particularly in the paired t-test result, a statistically significant difference was detected with the test group of mandibles only between Visit 3 and Visit 5 (Table 4).
With the maxillae, however, no statistically significant difference was found between the test group and the control group for all different periods (Table 5). During the observation of the test group and the control group one year later, the survival rate and success rate were found to be 100%.
The surface of root-type implant has shifted from a smooth and glossy surface to a rough one. There are several ways to form a rough surface. The hydroxyapatite process method has drawn attention for its bio-affinity and attainment of early stability. Hydroxyapatite itself is superior in terms of adhesion with bone and stabillity; in the long run, however, it is exfoliated from implant fixtures. This causes the separation of fixtures from the bone-hydroxyapatite attachment, thereby resulting in the failure of implant treatment.12
Because this study is clinical test, roughened surface fixture was used instead of machined surface fixture. The RBM process is method to roughen the surface of the implant. CMP coating process makes just a little change of Roughness.
The CMP coating is known for its many merits compared to the plasma-sprayed hydroxyapatite (HA) coating. Its coated layer is less than 3 µm thick; unlike the hydroxyapatite coating wherein molecular exfoliation occurs due to TCP dissolution, the CMP coating does not have the same problem of exfoliation of the coated layer from implant fixtures. As an improved method of HA coating, which exfoliates from fixtures, the RBM surface is widely used for implant surface processing. According to recent animal testing, the bone-implant contact of CMP-coated fixtures had greater value than machined fixtures. Such difference was statistically significant.12 This test did not use two different types of implants per person. As such, the comparison was not considered precise. Note, however, that no statistically significant difference was found compared to the currently commercially available RBM-processed fixtures. Therefore, the short-term use of CMP coating is clinically acceptable. Note, however, that this study did not have more frequent routine checkups within 2-3 months since the difference between RBM-processed fixtures and CMP-coated ones appears within 2-3 months of placement as reported through some studies and animal tests.28 Thus, this is something that can be improved in the future. The measurement of mobility using Perio-test, which was also used by this study was introduced by Schulte.28 The Perio-test is a device that figuratively displays the contact time measured through the repetition of instant contact with a measurement stick and an abutment. The Perio-test value ranges from -8 up to +50; its measurement method is simple, and it has been widely used in measuring the osseointegration of implants. Lately, the resonance frequency analysis (RFA) method has been used. Note, however, that measuring stability after prosthetic mounting requires the removal of the prosthesis, which is inconvenient. Although some studies found that the measured values of the Perio-test depended on the measuring direction and angle,29 repetitive measurements enabled obtaining stable values; even after prosthetic mounting, the test was regarded as an appropriate method to evaluate implant stability. This study obtained acceptable Perio-test values from both the control group and the test group. No statistically significant difference was found, however. In terms of the absorption of marginal bone, both the test and control groups yielded a clinically acceptable range of values. No statistically significant difference was observed between the two groups. Although the difference was not statistically significant, the test group showed more absorption. This was believed to be due to the varying individual implants contained in the groups. In the control group, female marginal bone accounted for 50%, which was greater compared to the test group. Wyatt and Zarb30 found during their observations one year after the implant load that the absorption level of male marginal bone was greater than the female one. Implant placement was considered successful when the cervical bone resorption measured for each implant did not exceed 1 mm for the first year.31 This study obtained acceptable marginal bone loss from both the control group and the test group.
In the one year study after the implant placement, no clinical difference was observed between RBM-processed and CMP-coated implants. A short-term, non-destructive clinical observation method is necessary to examine the changes that occur in the early stage. Although this study was not a long-term one, a decent clinical result on CMP-coated implants was obtained. Furthermore, since CMP-coated fixtures histologically had better bone reaction than RBM-processed ones in some tests and studies, a long-term study is expected to offer a better result and require an improved evaluation method.
The result of the evaluation performed in one year after the placement of CMP-coated fixtures showed that they were comparable to conventional RBM-processed fixtures. A non-destructive clinical observation method that will examine the early changes should be developed. A long-term study is also required to determine consistent stability and to identify the difference from conventional implant fixtures.
Figures and Tables
Table 2
Mean and standard deviation and statistical analysis of Perio-test value one year after fixture installation

Table 3
Mean and standard deviation and statistical analysis of marginal bone loss at different check point of time
References
1. Albrektsson T, Sennerby L. State of the art in oral implants. J Clin Periodontol. 1991; 18:474–481.
2. Eckert SE, Parein A, Myshin HL, Padilla JL. Validation of dental implant systems through a review of literature supplied by system manufacturers. J Prosthet Dent. 1997; 77:271–279.
3. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–170.
4. Carlsson L, Röstlund T, Albrektsson B, Albrektsson T. Removal torques for polished and rough titanium implants. Int J Oral Maxillofac Implants. 1988; 3:21–24.
5. Shin HS, Kim YS, Shin SW. Effects of various surface treatments for titanium on surface micro roughness, static wettability, fibronectin adsorption. J Korean Acad Prosthodont. 2006; 44:443–454.
6. Chen J, Tong W, Cao Y, Feng J, Zhang X. Effect of atmosphere on phase transformation in plasma-sprayed hydroxyapatite coatings during heat treatment. J Biomed Mater Res. 1997; 34:15–20.
7. Block MS, Kent JN, Kay JF. Evaluation of hydroxylapatite-coated titanium dental implants in dogs. J Oral Maxillofac Surg. 1987; 45:601–607.
8. Darimont GL, Cloots R, Heinen E, Seidel L, Legrand R. In vivo behaviour of hydroxyapatite coatings on titanium implants: a quantitative study in the rabbit. Biomaterials. 2002; 23:2569–2575.
9. Yamashita K, Kanazawa T. Materials Science Monographs: Inorganic phosphate materials. Tokyo: Elsevier;1989. p. 221–246.
10. Lee YM, Seol YJ, Lim YT, Kim S, Han SB, Rhyu IC, Baek SH, Heo SJ, Choi JY, Klokkevold PR, Chung CP. Tissue-engineered growth of bone by marrow cell transplantation using porous calcium metaphosphate matrices. J Biomed Mater Res. 2001; 54:216–223.
11. Park EK, Lee YE, Choi JY, Oh SH, Shin HI, Kim KH, Kim SY, Kim S. Cellular biocompatibility and stimulatory effects of calcium metaphosphate on osteoblastic differentiation of human bone marrow-derived stromal cells. Biomaterials. 2004; 25:3403–3411.
12. You CK, Yeo IS, Kim MD, Eom TK, Lee JY, Kim SK. Characterization and in vivo evaluation of calcium phosphate coated cp-titanium by dip-spin method. Curr Appl Phys. 2005; 5:501–506.
13. Yeo IS, Han JS, Yang JH. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J Biomed Mater Res B Appl Biomater. 2008; 87:303–311.
14. The Korean academy of oral and maxillofacial implantology. Atlas of implant terminology. 1st ed. Seoul: Deahan Publishing;2003. p. 15–16.
15. Kim YK, Park HS, Jung SM. Implant problem solution. Vol 2: Surgery and Prosthetic problem solution. 1st ed. Seoul: Deahan Publishing;2003. p. 9–10.
16. Roos J, Sennerby L, Lekholm U, Jemt T, Gröndahl K, Albrektsson T. A qualitative and quantitative method for evaluating implant success: a 5-year retrospective analysis of the Brånemark implant. Int J Oral Maxillofac Implants. 1997; 12:504–514.
17. Buser D, Weber HP, Lang NP. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin Oral Implants Res. 1990; 1:33–40.
18. Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP, Belser UC, Lang NP. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997; 8:161–172.
19. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986; 1:11–25.
20. Buser D, Brägger U, Lang NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res. 1990; 1:22–32.
21. Noack N, Willer J, Hoffmann J. Long-term results after placement of dental implants: longitudinal study of 1,964 implants over 16 years. Int J Oral Maxillofac Implants. 1999; 14:748–755.
22. Roos J, Sennerby L, Lekholm U, Jemt T, Gröndahl K, Albrektsson T. A qualitative and quantitative method for evaluating implant success: a 5-year retrospective analysis of the Brånemark implant. Int J Oral Maxillofac Implants. 1997; 12:504–514.
23. Brånemark PI, Zarb GA, Albrektsson T. Tissue-integrated prostheses: Osseointegration in clinical dentistry. In : Lekholm U, Zarb GA, editors. Patient selection. Chicago: Quintessence;1985. p. 199–209.
24. Sekine H, Komiyama Y, Hotta H, Yoshida K. Mobility characteristics and tactile sensitivity of osseointegrated fixture-supporting systems. In : Van Steeberghe D, editor. Tissue intergration in oral maxillofacial reconstruction. Amsterdam: Elsevier;1986. p. 236–332.
25. Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987; 2:145–151.
26. Curtis DA, Sharma A, Finzen FC, Kao RT. Occlusal considerations for implant restorations in the partially edentulous patient. J Calif Dent Assoc. 2000; 28:771–779.
27. Weinberg LA. Reduction of implant loading with therapeutic biomechanics. Implant Dent. 1998; 7:277–285.
28. Schulte W, d'Hoedt B, Lukas D, Maunz M, Steppeler M. Periotest for measuring periodontal characteristics--correlation with periodontal bone loss. J Periodontal Res. 1992; 27:184–190.
29. Faulkner MG, Giannitsios D, Lipsett AW, Wolfaardt JF. The use and abuse of the Periotest for 2-piece implant/abutment systems. Int J Oral Maxillofac Implants. 2001; 16:486–494.
30. Wyatt CC, Zarb GA. Bone level changes proximal to oral implants supporting fixed partial prostheses. Clin Oral Implants Res. 2002; 13:162–168.
31. Schwartz-Arad D, Yaniv Y, Levin L, Kaffe I. A radiographic evaluation of cervical bone loss associated with immediate and delayed implants placed for fixed restorations in edentulous jaws. J Periodontol. 2004; 75:652–657.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download