Abstract
PURPOSE
The purpose of this case series was to evaluate the effect of guided bone regeneration using demineralized allogenic bone matrix with calcium sulfate.
MATERIALS AND METHODS
Guided bone regeneration using Demineralized Allogenic Bone Matrix with Calcium Sulfate (AlloMatrix™, Wright. USA) was performed at the time of implant placement from February 2010 to April 2010. At the time of the second surgery, clinical evaluation of bone healing and histologic evaluation were performed. The study included 10 patients, and 23 implants were placed. The extent of bony defects around implants was determined by measuring the horizontal and vertical bone defects using a periodontal probe from the mesial, distal, buccal, and lingual sides and calculating the mean and standard deviation of these measurements. Wedge-shaped tissue samples were obtained from 3 patients and histologic examination was performed.
RESULTS
In clinical evaluation, it was observed that horizontal bone defects were completely healed with new bones, and in the vertical bone defect area, 15.1% of the original defect area remained. In 3 patients, histological tests were performed, and 16.7-41.7% new bone formation was confirmed. Bone graft materials slowly underwent resorption over time.
Since Urist and Strates1 introduced the concept of osteoinduction using bone morphogenic protein (BMP) present in cortical bones in 1965, numerous studies have investigated allogenic bone graft and related materials. Recently, numerous commercialized allogenic bone graft materials have been used in clinics. Allogenic bones have been reported to exhibit healing by osteoinduction and osteoconduction after graft. Osteoinductive activity is influenced by the content of BMP and Type I collagen contained in allogenic bones. In other words, inappropriately treated allogenic bones may not have osteoconductive function.2-5 In addition, healing may be affected by the age of the donor, the area of bone harvest, and the composition of bone (e.g., whether it is cortical bone or cancellous bone).6 The osteoconductive capacity of demineralized freeze-dried allogenic bones is dependent on bone morphogenic proteins contained in demineralized bone matrix (DBM), and as the content of DBM is increased, the effect is notably increased. In experiments that compared the effectiveness of bone formation in cases with 100% as compared to 17% DBM formation, the 100% cases showed markedly superior results with respect to the formation of neovasculature, osteoblastic activity, and cartilage and bone formation.5,7 Therefore, when clinicians select allogenic bone graft materials, they should select allogenic bone products containing a large amount of demineralized bone matrix that were treated at approved tissue banks. In addition, if the capacity for tissue regeneration is similar, clinicians should select products that can be manipulated readily and inexpensively. In this study, we applied AlloMatrix™ which is an allogenic product that serves as a carrier of calcium sulfate and was recently introduced in the dental field to guide bone regeneration. The purpose of this study was to evaluate the effect of guided bone regeneration using demineralized allogenic bone matrix with calcium sulfate. Bone healing capacity and clinical availability were assessed by clinical as well as histological observation.
This case series study was conducted on patients who underwent guided bone regeneration around the implant using Demineralized Allogenic Bone Matrix with Calcium Sulfate (AlloMatrix™, Wright Medical Technology Inc., Arlington, TN, USA) at the Seoul National University Bundang Hospital from February 2010 to April 2010. Patients with controlled systemic diseases and smokers were included in this study. The study included 10 patients, and 23 implants were placed. The age of the patients was 51-83 years, with an average of 66.8 years. Six patients were male and 4 patients were female. With regard to patients with controlled systemic diseases, 3 patients had hypertension, 1 patient had cardiac disease, 1 patient had Parkinson's disease, and 1 patient had hepatitis. One smoker was included. The study was performed after obtaining approval from the IRB, Bundang Seoul National University Hospital (B-1012-118-101). We have read the Helsinki Declaration and have followed the guidelines in this investigation.
After implant placement, the volumes of horizontal and vertical bone defects in the vicinity of implants were measured by a periodontal probe. Cover screws were connected, and bone graft was subsequently performed. The AlloMatrix™ powder was mixed with liquid using plastic spatulas; molding using fingers was performed in the defect area; and the first suture was performed on the flaps. After healing period of 3-6 months, the implant and the bone graft area were exposed by second surgery. The volume of bone defect in the vicinity of the implant was measured by a periodontal probe, and tissue samples were obtained from some patients after obtaining their consent. Healing abutments were connected, and conventional prosthesis treatments were initiated. Implants were placed 7 in the maxillary molar, 6 in the mandibular molar, 4 in the maxillary premolar, 2 in the mandibular premolar, 2 in the maxillary anterior teeth, and 2 in the madibular anterior teeth. All implants (Osstem TS III SA, GS III RBM; Osstem Implant Co., Busan, Korea) placed were tapered in shape; implants 4 mm in diameter and 13 mm in length were placed in most cases. Guided bone regeneration using AlloMatrix™ and implant placement were performed simultaneously. In 3 patients, sinus lifting was performed simultaneously. In 1 patient, hroziontal ridge augmentation was performed. Other bone graft materials have been used at 2 patients with sinus lifting performed and 1 patient with ridge augementation performed: one patients used xenogenic bone (XenoBT; Korea Tissue Bank, Seoul, Korea); one patient used AutoBT (Korea Tooth Bank, Seoul, Korea) and xenogenic bone (BioOss, Geistlich Pharma AG, Wolhausen, Switzerland) and one patient used allogenic bone block (Osteo.in, Seoul, Korea). Barrier membranes were not used in 9 patients. Nonetheless, collagen membranes (Ossix plus; Orapharma Inc., Louis Drive Warminster, PA, USA) were used in 1 patient who required a large volume of guided bone regeneration.
The extent of bony defects around implants was determined by measuring the horizontal and vertical bone defects using a periodontal probe from the mesial, distal, buccal, and lingual sides and calculating the mean of these measurements (unit: millimeter). At the time of the second surgery, the extent of the bone defect around the implant was measured using the same method. The ratio of the bone defect area remaining at the time of the second surgery to the initial bone defect area was calculated (bone defect after GBR/primary defect × 100), and the ratios of the two groups were compared. The mean and standard deviation of all values were calculated.
Tissue samples were taken on patients who signed a consent form after having received an explanation as to the purpose of histological tests. These samples were obtained at the time of the second surgery using a #15 surgical blade. Wedge-shaped tissue samples were obtained from 3 patients. Samples were immediately fixed in 10% formalin solution for 24 hours and decalcified with Calci-Clear Rapid™ (National Diagnostics, Atlanta, Georgia, USA) for 12 hours. Decalcified samples were washed with running water and treated with an automatic tissue processor (Hypercentre XP, Shandon, Cheshire, UK). After paraffin embedment, the samples were sectioned to 4-5 µm in thickness, stained with hematoxylin-eosin, and examined under a light microscope. Histomorphometric evaluation, performed by a single pathologist, included use of the computer-assisted Visus Image Analysis System (Image & Microscope Technology, Daejon, Korea). New bone formation ratio was measured as follows: lamellar bone: woven bone: residual graft material.
The initial stability of implants measured by the Osstell Mentor (Integration Diagnostics AB, Göteberg, Sweden) was 7-86 ISQ (mean: 61.6), and the second stability value was 56-87 ISQ (mean: 73.3). The healing period required for installation of the upper prosthesis in the maxilla was 4.5 - 8.0 months (mean: 5.5); the healing period required for installation in the mandible was 1.5-3 months (mean: 2.3). The final prosthesis treatment was completed in 9 patients, including 20 implants. In one patient who received 3 implants, a second surgery was completed, and prosthesis treatments are ongoing. No case with early implant failure was observed.
The horizontal bone defect volume after implant placement measured by a periodontal probe was 1.72 ± 0.73 mm, and the vertical bone defect volume was 3.28 ± 2.43 mm. At the time of second surgery, complete bone healing was achieved in the horizontal bone defect area, and the vertical bone defect volume was 0.53 ± 1.24 mm. The ratio of the bone defect volume remaining after guided bone regeneration to the initial bone defect area was assessed. The vertical bone defect area was 15.1%, and the horizontal bone defect area was shown to be 0%.
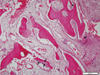
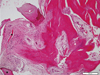
Tissues samples were obtained from the mandibular molar area of a 51-year-old female patient after 3 months. Revitalization of the implant chips with some degree of woven formation around the implant chips was noted. Absorbing implant chips were found in the fibrous tissue. Neither inflammatory cell infiltration nor granulomatous reaction was noted. Histomorphometric analysis was performed. New bone formation was 16.7%, and the ratio of lamellar bone to woven bone to residual graft materials was 0:43:57 (Fig. 1). Tissue samples were obtained from the maxillary left molar area of a 68-year-old male patient 4.5 months after GBR. Active woven bone formation was identified around the implant chips. Some areas showed bony trabeculae forming anastomosis. Neither inflammatory cell infiltration nor granulomatous reaction was noted. New bone formation was 41.7%, and the ratio of lamellar bones to woven bones to residual graft material was 0:89:11 (Fig. 2). Tissue samples were obtained from the maxillary left first molar area of a 75-year-old male patient after 5.5 months. Active woven bone formation was identified around the implant chips. Most of the implant chips were absorbed, but some particles were isolated in the fibrous tissue. Neither inflammatory cell infiltration nor granulomatous reaction was noted. New bone formation accounted for 27.3% of the tissue area quantified; the lamellar bone: woven bone: residual graft material ratio was 0:88:12 (Fig. 3).
Allogenic bones are classified into fresh allograft, frozen bones, frozen irradiated bones, freeze-dried non-demineralized bones, lyophilized bones (FDBA), demineralized freeze-dried allogeneic bone (DFDBA), and demineralized bone matrix (DBM) according to treatement methods. Nevertheless, frozen irradiated bones, freeze-dried non-demineralized bones, and demineralized freeze-dried allogeneic bones have been used most frequently in the clinic.8 The osteoinductive capacity of allogenic bones is influenced by bone storage treatment methods (deep frozen, freeze-dried, etc.), bone mineral treatment methods (calcified, surface decalcified, or fully decalcified), the type of bones used (cortical, cancellous), the physical shape of graft materials (powder, chips, blocks, laminated strips), the condition of the receiver area, the type of animals used to test the capacity of bone graft materials, the origin of graft materials, the age of the donor, the particle size of graft materials, and sterilization methods.4-6,8 Published reports state that 10-15% of DBM is not osteoinductive. It is therefore imperative that each lot be evaluated prior to use to ensure osteoinductive potential.9 All DBM lots that are incorporated into AlloMatrix™ putty should be tested for osteoinductivity using a proprietary in vitro bio-assay that measures the direct response of the DBM to human boneforming cells.10 Therefore, it is important to select high-quality allogenic bones prepared by approved tissue banks.
The osteoinductive effects of demineralized freeze-dried allogenic bones are dependent on the amount of BMP contained in DBM. As the level of DBM increases, the effect of osteoinduction is notably increased. To obtain clinically acceptable bone-healing effects, the DBM content should account for at least 20% of the weight/weight ratio.7 Regarding the content according to the weight/weight ratio of allogenic products used widely in clinics, the content suggested by the manufacturer is 19.5% for Orthoblast II® (Isotis OrthoBiologics, Irvine USA); 24-33% for Regenaform® (Regeneration Technologies Inc., Alachua, FL, USA); and 27-35% for DBX® (MTF, Edison, NJ, USA). The DMB content in good-quality allogenic bones prepared by approved tissue banks is comparable, and they show good healing by osteoinduction.9,10 Therefore, clinicians favor products that can be easily manipulated and are inexpensive. They are commercialized as putty, gel, by adding various carriers. Carboxymethylcellulose (CMC), hyaluronic acid, glycerol, starch, reversephase medium (RPM), calcium sulfate, and gelatin represent typical carriers.8,11
The recently commercialized AlloMatrix™ is a DBM-injectable putty derived from human bones. Its DBM content is high, 86% by volume, and it contains various bone growth factors, such as BMP-2, BMP-4, IGF-1, and TGF-β1; excellent healing by osteoinduction has been demonstrated. In addition, the collagen matrix contained in DBM plays the role as an osteoconductive scaffold. The product is a powder containing a mixture of freeze-dried DBM and surgical-grade calcium sulfate as carriers. Immediately prior to use, it is mixed by clinicians with the mixing solution contained in the products as putty forms. AlloMatrix™ can be readily manipulated, well adapted to the surgery area, and does not require the use of barrier membrane because graft materials themselves function as barriers.12-14 Prehydrating proteins may degrade BMP over time. AlloMatrix™ is provided in a powder form with freeze-dried DBM and surgical grade calcium sulfate. By choosing not to pre-hydrate the DBM, bone morphogenetic proteins are preserved until the point of use. The powdered form also allows for a longer shelf life.15
In this study, clinical evaluation of bone healing after GBR was performed on 10 patients; histological tests were performed in 3 patients. The bone defect volume measured immediately after implant placement confirmed that at the time of the second surgery, significant bone healing was achieved. Almost 100% of the horizontal bone defect volume exhibited successful bone healing. Nevertheless, only approximately 15.1% of the vertical bone defect volume remained in comparison with the initialperiod. Histological tests revealed that between 3-6 months, relatively good new bone formation was achieved, and the temporal pattern of residual bone graft material resorption was observed. Nonetheless, between 3-6 months, the formation of lamellar bones was not observed; it is estimated that bone maturation was delayed.
The limitations of our study are that the number of subject patients is small and that histological tests were performed on only 3 patients. Therefore, it was not possible to perform histomorphometric analysis accurately. Four patients used other bone graft materials in combination. A variety of bone graft material can affect the bony healing in this study. In addition, the bone healing pattern of the maxillary and mandibular bone defect areas was different. It is therefore necessary to comprehensively analyze bone healing patterns of the maxillary and mandibular defect areas in more study subjects in the future.
Figures and Tables
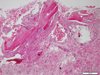 | Fig. 1Revitalization of the implant chips (white asterisks) with some degree of woven formation around the implant chips. Absorbing implant chips (black asterisks) were found in the fibrous tissue. |
References
1. Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971; 50:1392–1406.
2. Han B, Tang B, Nimni ME. Quantitative and sensitive in vitro assay for osteoinductive activity of demineralized bone matrix. J Orthop Res. 2003; 21:648–654.
3. Schwartz Z, Mellonig JT, Carnes DL Jr, de la Fontaine J, Cochran DL, Dean DD, Boyan BD. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1996; 67:918–926.
4. Takikawa S, Bauer TW, Kambic H, Togawa D. Comparative evaluation of the osteoinductivity of two formulations of human demineralized bone matrix. J Biomed Mater Res A. 2003; 65:37–42.
5. Ziran B, Cheung S, Smith W, Westerheide K. Comparative efficacy of 2 different demineralized bone matrix allografts in treating long-bone nonunions in heavy tobacco smokers. Am J Orthop (Belle Mead NJ). 2005; 34:329–332.
6. Traianedes K, Russell JL, Edwards JT, Stubbs HA, Shanahan IR, Knaack D. Donor age and gender effects on osteoinductivity of demineralized bone matrix. J Biomed Mater Res B Appl Biomater. 2004; 70:21–29.
7. Zhang M, Powers RM Jr, Wolfinbarger L Jr. A quantitative assessment of osteoinductivity of human demineralized bone matrix. J Periodontol. 1997; 68:1076–1084.
8. Kim YK, Kim SG, Lee BG. Bone Graft and Implant. Bone biology and bone graft material. Vol. 1. Seoul, Korea: Daehan Narae Pub Co;2007. p. 140–142.
9. Wilkins RM, Kelly CM, Giusti DE. Bioassayed demineralized bone matrix and calcium sulfate: use in bone-grafting procedures. Ann Chir Gynaecol. 1999; 88:180–185.
10. Adkisson HD, Strauss-Schoenberger J, Gillis M, Wilkins R, Jackson M, Hruska KA. Rapid quantitative bioassay of osteoinduction. J Orthop Res. 2000; 18:503–511.
11. Cheung S, Westerheide K, Ziran B. Efficacy of contained metaphyseal and periarticular defects treated with two different demineralized bone matrix allografts. Int Orthop. 2003; 27:56–59.
12. Gitelis S, Piasecki P, Turner T, Haggard W, Charters J, Urban R. Use of a calcium sulfate-based bone graft substitute for benign bone lesions. Orthopedics. 2001; 24:162–166.
13. Turner TM, Urban RM, Gitelis S, Kuo KN, Andersson GB. Radiographic and histologic assessment of calcium sulfate in experimental animal models and clinical use as a resorbable bone-graft substitute, a bone-graft expander, and a method for local antibiotic delivery. One institution's experience. J Bone Joint Surg Am. 2001; 83-A:8–18.
14. Kelly CM, Wilkins RM, Gitelis S, Hartjen C, Watson JT, Kim PT. The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001; 382:42–50.
15. Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997; 14:969–975.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download