Abstract
PURPOSE
The cortical bone thickness on the anterior region is important for achieving implant stability. The purpose of this study was to examine the thickness of the cortical and cancellous bones on the anterior region of the maxilla and mandible.
MATERIALS AND METHODS
Twenty-five cadaver heads were used (16 male and 9 female; mean death age, 56.7 years). After the long axis of alveolar process was set up, it was measured in 5 levels starting from 2 mm below the cementoenamel junction (L1) at intervals of 3 mm. All data was analysed statistically by one-way ANOVA at the .05 significance level.
RESULTS
The cortical bone thickness according to measurement levels in both the labial and lingual sides increased from L1 to L5, and the lingual side below L3 was significantly thicker than the labial side on the maxilla and mandible. In particular, the labial cortical bone thickness in the maxilla was the thinnest compared to the other regions. The cancellous bone thickness according to measurement levels increased from L1 to L5 on the maxilla, and on the mandible it was the thinnest at the middle level of the root.
The anterior teeth are an important factor in dental and facial esthetics.1 At this time, in the anterior region they are difficult for both stabilization of the implant fixture and aesthesis of the restoration because they have narrower alveolar ridge and thinner cortical bone than in the posterior region.2,3 In addition, the thickness of the cortical bone has a larger influence on the initial stabilization than the length of the implant fixture in an edentulous region. Therefore, information on the cortical and cancellous bone thickness in the labial and lingual sides, especially the anterior part, is the key to successful dental implantation.4
Many researchers have evaluated the thickness of labial cortical bone using various methods in the anterior region. Above all, computed tomography (CT) is used widely to measure the alveolar bone for preoperative evaluation before dental prosthetic and orthodontic treatment because it can measure a large number of the samples and various age groups. Using a CT, Flanagan5 compared the thickness of the labial and lingual cortical bone on the edentulism, and Swasty et al.6 measured the thickness of the mandibular alveolar bone in various age groups. In addition, Deguchi et al.7 and Lim et al.8 evaluated the thickness of the buccal and lingual cortical bone on the posterior region using CT for the implantation of mini-screws.
Recently, micro-CT is often used for measuring the bone structures because it is convenient, noninvasive tool and has the higher resolution compared to conventional CT image.9 However, until now, most of these researches have been done by using the radiographic methods such as CT. Despite the limited number of the cadaver samples, few studies by direct manual measurement have evaluated the thickness of cortical and cancellous bone on cadavers.
Therefore, this study was carried out to evaluate the cortical and cancellous bone thickness at the midline and interdental areas of the tooth in the anterior region to provide anatomic information for dental implantation on dentate Korean cadavers.
Twenty-five cadaver heads were used (16 male and 9 female), whose age at death ranged from 40 to 90 years (mean age: 56.7 years). To measure the cortical and cancellous bone thickness of the anterior region in dentate Korean cadavers, the maxilla and mandible with all anterior teeth including canine were chosen.
All specimens were decalcified in a decalcification solution (a mixture of 8 N formic acid + 1 N sodium formate) for 1 month, and then neutralized in distilled water for 12 hours. They were cut along the midline and interdental (distal surface) areas of each anterior tooth from the labial side to the lingual side using a microtome blade (Feather Co., Osaka, Japan) at total of 6 planes: midline area of central incisor (CI-mid), interdental area between CI and lateral incisor (CI-ID), midline area of lateral incisor (LI-mid), interdental area between LI and canine (LI-ID), midline area of canine (C-mid) and interdental area between C and the first premolar (C-ID). Each sectioned specimen was scanned using a scanner (HP Scanjet G4050, Hewlett Packard Co., Houston, Tex, USA) with ruler to correct the size at the same time, and the scanned images were measured using Adobe Photoshop CS3 ver 10 (Adobe, CA, USA) to a 0.01 mm level.10
On each scanned image, the long axis of the alveolar process was set to connect the center point of the cementoenamel junction (CEJ) with the center point of extension line passing through the 14 mm point below the CEJ even with the CEJ (Fig. 1). Considering the mean root length of incisors in the maxilla and mandible (12.5 - 14 mm),11 the cortical and cancellous bone thickness on both the labial and lingual sides was measured in 5 levels starting from 2 mm below the CEJ (L1) at intervals of 3 mm to root apex area. At the midline area of the tooth, the total cancellous bone thickness including the root thickness was determined (Fig. 2). The cadavers used in this study were in the elderly and degree of absorption of the alveolar process was different regardless of the dentate conditions. Therefore, the cortical bone thickness in L1 was referred in the results only and excluded from the discussion. All measurements were carried out by two investigators. The interobserver agreement was high (P=.672), therefore, the average of the 2 measurements was used as the final measurement.
The inter-observer difference was analysed by one-way ANOVA. All measurements were reported as the mean±SD. The difference between right and left sides was analysed by one-way ANOVA, and there was no significant difference so each side measurement was counted as the same group. The cortical bone thickness in the midline area was compared t that of interdental area at the same level, and then evaluated with a post-hoc Scheffé comparison. As a result, no significant difference was found in the cortical bone thickness between the midline and interdental areas in both the labial and lingual sides. Therefore, the average value of the 2 measurements was calculated to compare the difference of the cortical bone thickness in the labial and lingual sides (Figs. 3, 4). All statistical analyses were performed using SPSS 12.0 (Chicago, IL, USA). P value <.05 was considered significant.
The cortical bone thickness of the midline area was thinnest in L2 and L3 of the C on both the labial and lingual sides, and thickest in the L2 and L3 of the CI. In addition, the cortical bone thickness increased from L1 to L5 in every tooth sites on both the labial and lingual sides. The labial cortical bone thickness in L1 and L2 near the alveolar crest was thicker than the lingual side, and the lingual sidein L3, L4, and L5 under the middle level of the root was thicker than the labial side. The cancellous bone thickness of the midline area varied according to the tooth sites and measurement levels (Table 1).
The cortical bone thickness of the interdental area was thinnest in the CI-ID on both the labial and lingual sides, and thickest in the C-ID among the tooth sites. As in the midline area, the cortical bone thickness increased from L1 to L5 in both the labial and lingual sides. The labial cortical bone thickness in L1 and L2 was thicker than the lingual side, and the lingual side in L3, L4, and L5 except the CI-ID was thicker than the labial side. The cancellous bone thickness of the interdental area varied according to the tooth sites and the measurement levels, and it seemed to become thinner at the middle level of the root (L2, L3, and L4) in the CI-ID and LI-ID (Table 2).
The cortical bone thickness of the midline area was thinnest in the CI on both labial and lingual sides, and thickest in the LI on the labial side and in the C on the lingual side among the tooth sites. As in the midline area in the maxilla, the cortical bone thickness increased from L1 to L5 in both labial and lingual sides, and the labial cortical bone thickness in L1 and L2 near the alveolar crest was thicker than the lingual side and the lingual side in L3, L4, and L5 under the middle level of the root was thicker than the labial side. The cancellous bone thickness of the midline area was thinnest in the CI, and thickest in the C among the tooth sites, and it became thinner at the middle level of the root (L3, L4) in all tooth sites (Table 3).
The cortical bone thickness of the interdental area was thinnest in the CI-ID on both labial and lingual sides, and thickest in the C-ID among the tooth sites. As like the midline area in the mandible, the cortical bone thickness increased from L1 to L5 in both the labial and lingual sides, and the lingual side under L2 was thicker than the labial side except for the L2 of LI-ID. The cancellous bone thickness of the interdental area was thinnest in the CI-ID, and thickest in the C-ID among the tooth sites. In addition, it became thinner at the middle level of the root (L3, L4) in all tooth sites (Table 4).
The average thickness of labial cortical bone according to the measurement levels on the maxilla was 0.12 ± 0.22 mm (L1), 0.40 ± 0.33 mm (L2), 0.47 ± 0.28 mm (L3), 0.54 ± 0.26 mm (L4), and 0.63 ± 0.27 mm (L5). The average thickness of lingual cortical bone according to the measurement levels on the maxilla was 0.02 ± 0.09 mm, 0.30 ± 0.37 mm, 0.55 ± 0.33 mm, 0.62 ± 0.31 mm, and 0.66 ± 0.32 mm, respectively (Fig. 3). The average thickness of labial cortical bone according to the measurement levels on the mandible was 0.13 ± 0.29 mm, 0.48 ± 0.32 mm, 0.56 ± 0.29 mm, 0.69 ± 0.31 mm, and 0.88 ± 0.31 mm, respectively. The average thickness of lingual cortical bone according to the measurement levels on the mandible was 0.05 ± 0.21 mm, 0.49 ± 0.43 mm, 0.89 ± 0.47 mm, 1.04 ± 0.41 mm, and 1.21 ± 0.42 mm, respectively (Fig. 4). On both the maxilla and mandible, the cortical bone thickness increased from L1 to L5 both the labial and lingual sides, and the labial cortical bone thickness in L1 and L2 was thicker than the lingual side, and the lingual side in L3, L4, and L5 was thicker than the labial side (Figs. 3, 4). On the maxilla, the labial and lingual cortical bone thickness showed-significant difference at all levels (Fig. 3), but on the mandible, there was significant difference only in L3, L4, and L5 under the middle level of the root (Fig. 4).
Using the average value of the midline and interdental area mentioned in the materials and methods, this study evaluated a diagram of the overall shape of the cortical and cancellous bone at each level on both maxilla and mandible (Fig. 5). As a result, the cancellous bone thickness increased from L1 to L5 on the maxilla, and was thickest at L2 and thinnest at L4 on the mandible (Fig. 5).
The sufficient thickness of the cortical bone is a primary factor in an initial fixation of implant placement.4,12 Although the thickness of the alveolar bone normally increases from the alveolar crest to the root apex, in the anterior region, the thickness of cancellous bone between the labial and lingual cortical bone is important because the width of the anterior alveolar process is narrower than that of molar.3 After tooth extraction, the alveolar bone undergoes the reparative resorption sequentially and, in particular, the labial cortical bone resorbs the first in the labial and alveolar crest directions.5 Therefore, the cortical and cancellous bone thickness in the anterior region should be an accurate assessment preoperatively for initial and successive implant stability.
In previous studies on the cortical bone thickness, Flanagan5 reported that the lingual cortical bone thickness was 2.33 mm, which was thicker than that of the labial side by 1.79 mm in edentulism, and the lingual side was always thicker than the labial side when bone absorption had already progressed. Katranji et al.12 reported that the cortical bone was thicker in the maxilla than the mandible, and the labial cortical bone was thicker than that of the lingual side in the dentate anterior region. In the maxilla the labial cortical bone thickness was 1.59 mm and the lingual cortical bone thickness was 1.95 mm, on the other hand, in the mandible they were 0.99 mm and 1.24 mm, respectively.
In the present study using dentate cadavers, the lingual cortical bone was thicker than the labial one below L3 in both the maxilla and mandible. In particular, the lingual cortical bone in the mandible was thick enough to support the bone by L3 (0.89 mm), L4 (1.04 mm) and L5 (1.21 mm). The mandible was thicker than the maxilla at all levels in both the labial and lingual sides. In addition, the labial cortical bone thickness in the maxilla was thin by L2 (0.40 mm), L3 (0.47 mm), L4 (0.54 mm) and L5 (0.63 mm). These results constrained the functional and esthetic recovery because a change in alveolar socket volume was observed in a certain amount for the first 8 weeks after extraction when prosthetic restoration and the labial side bone of the extraction socket is absorbed more than the lingual side.13,14 Therefore, for successful prosthetic restoration and implant placement after extraction, it is important to predict the initial absorption with consideration of the thinner labial cortical bone around the alveolar crest. At this time, in 6 months after implant placement the mean vertical bone resorption from the platform of implant was 1.32 ± 0.86 mm, and the residual labial bone thickness to implant fixture was 1.91 ± 0.45 mm. Therefore, the labial bone thickness more than 1.91 mm could prevent a failure of implant placement that could result from severe labial bone resorption.15
In particular, to solve the problem caused by the resorption of paper-thin labial cortical bone after extraction, clinicians recommended the placement of an immediate implant and the use of graft material as a remedy.16-18 Yoo et al.19 reported that the change of bone level in the alveolar crest was a recommendable level at an immediate implant placement after extraction, and the alveolar crest was absorbed more in the mandible than in the maxilla after immediate placement. Although full recovery occurred without guided bone regeneration in an immediate implant placementwhere the bone loss around the implant was small, Nevins et al.18 recommended the guided bone regeneration using graft materials because the thin labial alveolar bone on the anterior maxilla was absorbed easily. Covani et al.17,20 reported that the alveolar bone distance between the labial and the lingual side was 8.1 mm and 5.8 mm in immediate and delayed implant placement, respectively, as the secondary surgery. In addition, when immediate implant placement after extraction was performed on the anterior region, the vertical bone loss around the implant did not cause esthetic side effects given that it was restored completely 6 months after implant placement through a bone regeneration process.
On the other hand, Araújo et al.21,22 and Cardaropoli23 reported that the interspace between the implant and alveolar socket wall disappeared as a result of bone regeneration and absorption of the alveolar crest. At this time, the vertical bone absorption on the anterior region was more at the labial side than the lingual side, and when that was compared between the immediate implant placement and placement on edentulous bone, the result was similar to each other. Therefore, an immediate implant placement did not guarantee better results. However, according to this study and previous reports, immediate implant placement after extraction and guided bone regeneration are recommended for stability and aesthetics because the labial cortical bone of the anterior maxilla is particularly thin and more esthetically exposed than the mandible.17,19,24-26
Clinicians need to consider not only the cortical bone thickness but also the cancellous bone thickness for good blood supply and, therefore, successful implant placement. Brånemark, who made dental implants popular, classified the remaining bone quality into 4 types. He said that class 2, which is thick cortical bone and a high density of cancellous bone, and class 3, which is thin cortical bone and a high density of cancellous bone, are suitable bone types for successful implant placement. In addition, Misch27 classified the bone quality into 5 types using the Houndsfield units (HU) for easy application by an objective standard.
In the present study, the cancellous bone thickness in the maxilla was thinnest in the LI-ID and thickest in the C-mid, and the average thickness increased from the alveolar crest (L1) to the root (L5). In the present study, the cancellous bone thickness in the maxilla was thinnest in the LI-ID and thickest in the C-mid, and the average thickness according to measurement levels increased from the alveolar crest (L1) to the root apex (L5). The root width of maxillary incisors became suddenly narrower from 6 mm below the CEJ,9 in the present study, the cancellous bone thickness was measured including the root width. Therefore, the increasing thickness of cancellous bone toward the root apex (L5) could be reflected practical increasing of cancellous bone.
The cancellous bone thickness in the mandible was thinnest in the CI-ID and thickest in the C-mid, and on each level, the average thickness was thickest at L2 (4.74 mm) and thinnest at L4 (4.21 mm). That is, unlike the cancellous bone of maxilla, the cancellous bone thickness on the mandible became narrower at the middle level of the root. Miyamoto et al.4 reported that the cortical bone thickness is more important in the early stages of implant placement than the length of the implant fixture for stability, and the cancellous bone/cortical bone ratio is also important in the placement area. Therefore, the cancellous bone thickness of middle level of the root should be considered at first to select the diameter of implant fixture on the anterior mandibular region. In addition, the cancellous bone thickness and bone quality are important for implant placement. More studies using micro CT will be needed to measure the entire volume and cancellous bone density.
In the present study, used cadavers, whose age at death ranged from 40 to 90 years, were the elderly. However, Swasty et al.6 measured the cortical bone thickness in the mandible using CT according to age, and reported that it was thinnest in the first decade and thickest in the fifth decade with a decrease thereafter. So, this measured data related to the cortical and cancellous bone thickness could be smaller than that of the young generation. Therefore, further studies are needed to reveal the change of cortical bone thickness by age with supplementing a number of cadavers.
The cortical bone thickness was thicker in the lingual side than the labial side both on the maxilla and mandible except for L1, L2 around the alveolar crest. In particular, the labial cortical bone thickness in the maxilla was thinnest compared to the other regions. In addition, the cancellous bone thickness in the maxilla increased to the root apex, and it was thinnest at the middle level of the root in the mandible. For implant placement on the anterior region, a careful evaluation and full knowledge on the thickness of cortical and cancellous bone are necessary, providing an anatomic guideline to clinicians.
Figures and Tables
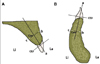 | Fig. 1Diagram showing a cross-section of the interdental area. A: maxilla, B: mandible. a: long axis of alveolar process, b: labial cortical bone thickness, c: lingual cortical bone thickness, Can: cancellous bone thickness, CEJ: cementoenamel junction, La: labial side, Li: lingual side. |
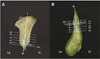 | Fig. 2Scanned image showing the 5 levels starting from 2 mm below the CEJ (L1) at intervals of 3 mm to the root apex area (L5). A: maxilla, B: mandible. a: long axis of alveolar process, CEJ: cementoenamel junction, La: labial side, Li: lingual side. L1: 2 mm below the CEJ, L2: 5 mm below the CEJ, L3: 8 mm below the CEJ, L4: 11 mm below the CEJ, L5: 14 mm below the CEJ. |
 | Fig. 3Diagram showing that the cortical bone thickness was compared in the labial and lingual sides at each level in the anterior region on the maxilla. *indicates statistical significance with a P<.05. |
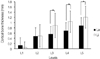 | Fig. 4Diagram showing that the cortical bone thickness was compared in the labial and lingual sides at each level in the anterior region on the mandible. *indicates statistical significance with a P<.05. |
 | Fig. 5Diagram showing the average thickness of cortical and cancellous bones at each level in the anterior region. A: maxilla, B: mandible. |
Table 1
Thickness (in millimeters; mean ± SD) of the cortical and cancellous bones at each level in the middle area of the tooth on the maxilla

Abbreviations; CI-mid: midline area of central incisor, LI-mid: midline area of lateral incisor, C-mid: midline area of canine, La: labial cortical bone thickness, Li: lingual cortical bone thickness, Can: cancellous bone thickness. L1: 2 mm below the CEJ, L2: 5 mm below the CEJ, L3: 8 mm below the CEJ, L4: 11 mm below the CEJ, L5: 14 mm below the CEJ.
Table 2
Thickness (in millimeters; mean ± SD) of cortical and cancellous bones at each level in the interdental area of the tooth on the maxilla

References
1. Ku JE, Yang HS, Yun KD. A morphometric analysis of maxillary central incisor on the basis of facial appearance in Korea. J Adv Prosthodont. 2012. 4:13–17.
2. Bernard JP, Schatz JP, Christou P, Belser U, Kiliaridis S. Long-term vertical changes of the anterior maxillary teeth adjacent to single implants in young and mature adults. A retrospective study. J Clin Periodontol. 2004. 31:1024–1028.
3. Buser D, Martin W, Belser UC. Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Implants. 2004. 19:43–61.
4. Miyamoto I, Tsuboi Y, Wada E, Suwa H, Iizuka T. Influence of cortical bone thickness and implant length on implant stability at the time of surgery-clinical, prospective, biomechanical, and imaging study. Bone. 2005. 37:776–780.
5. Flanagan D. A comparison of facial and lingual cortical thicknesses in edentulous maxillary and mandibular sites measured on computerized tomograms. J Oral Implantol. 2008. 34:256–258.
6. Swasty D, Lee JS, Huang JC, Maki K, Gansky SA, Hatcher D, Miller AJ. Anthropometric analysis of the human mandibular cortical bone as assessed by cone-beam computed tomography. J Oral Maxillofac Surg. 2009. 67:491–500.
7. Deguchi T, Nasu M, Murakami K, Yabuuchi T, Kamioka H, Takano-Yamamoto T. Quantitative evaluation of cortical bone thickness with computed tomographic scanning for orthodontic implants. Am J Orthod Dentofacial Orthop. 2006. 129:721.
8. Lim WH, Lee SK, Wikesjö UM, Chun YS. A descriptive tissue evaluation at maxillary interradicular sites: implications for orthodontic mini-implant placement. Clin Anat. 2007. 20:760–765.
9. Kim JH, Lee JG, Han DH, Kim HJ. Morphometric analysis of the anterior region of the maxillary bone for immediate implant placement using micro-CT. Clin Anat. 2011. 24:462–468.
10. Park HD, Min CK, Kwak HH, Youn KH, Choi SH, Kim HJ. Topography of the outer mandibular symphyseal region with reference to the autogenous bone graft. Int J Oral Maxillofac Surg. 2004. 33:781–785.
11. Shin JW. Dental anatomy. 2010. 3rd ed. Seoul: DaehanNarae Publishing, Inc.;61–99.
12. Katranji A, Misch K, Wang HL. Cortical bone thickness in dentate and edentulous human cadavers. J Periodontol. 2007. 78:874–878.
13. Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003. 30:809–818.
14. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005. 32:212–218.
15. Cho YB, Moon SJ, Chung CH, Kim HJ. Resorption of labial bone in maxillary anterior implant. J Adv Prosthodont. 2011. 3:85–89.
16. Covani U, Cornelini R, Barone A. Bucco-lingual bone remodeling around implants placed into immediate extraction sockets: a case series. J Periodontol. 2003. 74:268–273.
17. Covani U, Cornelini R, Barone A. Vertical crestal bone changes around implants placed into fresh extraction sockets. J Periodontol. 2007. 78:810–815.
18. Nevins M, Camelo M, De Paoli S, Friedland B, Schenk RK, Parma-Benfenati S, Simion M, Tinti C, Wagenberg B. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006. 26:19–29.
19. Yoo RH, Chuang SK, Erakat MS, Weed M, Dodson TB. Changes in crestal bone levels for immediately loaded implants. Int J Oral Maxillofac Implants. 2006. 21:253–261.
20. Covani U, Bortolaia C, Barone A, Sbordone L. Bucco-lingual crestal bone changes after immediate and delayed implant placement. J Periodontol. 2004. 75:1605–1612.
21. Araújo MG, Sukekava F, Wennström JL, Lindhe J. Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005. 32:645–652.
22. Araújo MG, Wennström JL, Lindhe J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin Oral Implants Res. 2006. 17:606–614.
23. Cardaropoli G, Lekholm U, Wennström JL. Tissue alterations at implant-supported single-tooth replacements: a 1-year prospective clinical study. Clin Oral Implants Res. 2006. 17:165–171.
24. Kan JY, Rungcharassaeng K. Immediate placement and provisionalization of maxillary anterior single implants: a surgical and prosthodontic rationale. Pract Periodontics Aesthet Dent. 2000. 12:817–824.
25. Wagenberg BD, Ginsburg TR.. Immediate implant placement on removal of the natural tooth: retrospective analysis of 1,081 implants. Compend Contin Educ Dent. 2001. 22:399–404. 406408
26. Juodzbalys G, Wang HL. Soft and hard tissue assessment of immediate implant placement: a case series. Clin Oral Implants Res. 2007. 18:237–243.
27. Misch CE. Density of bone: effect on treatment plans, surgical approach, healing, and progressive boen loading. Int J Oral Implantol. 1990. 6:23–31.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download