Abstract
PURPOSE
Finite element study on the effect of abutment length and material on implant bone interface against dynamic loading.
MATERIALS AND METHODS
Two dimensional finite element models of cylinderical implant, abutments and bone made by titanium or polyoxymethylene were simulated with the aid of Marc/Mentat software. Each model represented bone, implant and titanium or polyoxymethylene abutment. Model 1: Implant with 3 mm titanium abutment, Model 2: Implant with 2 mm polyoxymethylene resilient material abutment, Model 3: Implant with 3 mm polyoxymethylene resilient material abutment and Model 4: Implant with 4 mm polyoxymethylene resilient material abutment. A vertical load of 11 N was applied with a frequency of 2 cycles/sec. The stress distribution pattern and displacement at the junction of cortical bone and implant was recorded.
RESULTS
When Model 2, 3 and 4 are compared with Model 1, they showed narrowing of stress distribution pattern in the cortical bone as the height of the polyoxymethylene resilient material abutment increases. Model 2, 3 and 4 showed slightly less but similar displacement when compared to Model 1.
CONCLUSION
Within the limitation of this study, we conclude that introduction of different height resilient material abutment with different heights i.e. 2 mm, 3 mm and 4 mm polyoxymethylene, does not bring about significant change in stress distribution pattern and displacement as compared to 3 mm Ti abutment. Clinically, with the application of resilient material abutment there is no significant change in stress distribution around implant-bone interface.
Stress distribution at the dental implant-cortical bone interface has been a major concern for researchers since the inception of dental implants. A lot research have been done in trying to reduce or redistribute the stress on the cortical bone and achieve stress pattern as close to natural tooth as possible. Kirsch et al.1,2 introduced the concept of resilient material between the implant fixture and superstructure. The concept of resilient material introduction derived by the fact, that the resilient material will be able to absorb and redistribute the stresses better, as compared to titanium abutment. A study by Achour et al.3 introduced an elastomeric material as stress breaker and concluded that the use of prosthetic material with lower stiffness was capable to diminish or to delay the loads transmitted to implants and to the bone.
However, studies by Holmes et al.4 and McGlumphy et al.5 concluded that introduction of resilient material abutment does not result in significant reduction of cortical bone stress. In another study, changing the shape of the resilient material abutment produced only slight changes in the stress distribution in the cortical bone.6 A study by Masaki et al.,7 in which they compared different resilient material abutment, concluded that there was no significant difference between the stresses. Although a lot of finite element analysis also has been done using resilient material abutment materials, all these are static analysis.4,6,8 Since physiological loads in oral function such as mastication, time varying movements such as tapping and grinding should be simulated in a dynamic analysis.
In a dynamic study by Morton et al.,9 in which they evaluated the bone strain, concluded that there is no substantial reduction in measurable bone strain while using polyoxymethylene resilient material abutment as compared to titanium abutment. Recently, due to development of computer technologies, cyclic loading is simulated, by finite element method. Therefore, in this experimental study, we compare the effect of different height polyoxymethylene resilient material abutment on the bone-implant interface, when compared with 3 mm titanium abutment using two dimensional dynamic finite element method.
In this experimental study, cylindrical dental implant was selected for stress distribution pattern and displacement study. The cylindrical dental implant has polyoxymethylene resilient material abutment as an integral component of the dental implant system. This is a 2-dimensional, isotropic, homogeneous and non-linear analysis. In this analysis, dental implant model was created and analyzed on MARC/Mentat (2005r3, MSC Software, USA). The dental implant has 18 mm height and 2.5 mm width. The dental implant consists of nine threads which are parallel, and triangular in shape. The dental implant neck is 2.5 mm in length. The dental implant is placed inside the cortical bone with 1mm mucosa covering the cortical bone. 'Model 1' has 3 mm of Titanium as abutment in the dental implant system while 'Model 2, 3 and 4' have heights of 2 mm, 3 mm, and 4 mm of polyoxymethylene resilient material abutment in the dental implant system, shown in Fig. 1. The mandibular bone is shown as rectangular block. The mandibular bone is 22.8 mm in height and 25 mm in width. The mandibular bone consists of 2.5 and 2.3 mm of cortical bone on the superior and the inferior surface respectively. The cancellous bone lies between the layers of cortical bone and is 18 mm in height. Model 1 dental implant and bone consist of 2015 nodes and 2052 elements, while nodes and elements in Model 2, 3 and 4 dental implants and bone are shown in Fig. 2. All elements are tetrahedral in shape as shown in Fig. 3.
The nodes on the neck region of the dental implant and side of the mandibular bone are fixed in X direction while the nodes on the lower surface of the mandibular bone are fixed in X and Y direction as shown in Fig. 3. The implant and resilient material make up first contact body while the cortical and cancellous bone along with oral mucosa make up the second contact body as shown in Fig. 4. The friction coefficient for the dental implant and mandibular bone is 0.3. The mechanical properties of the bone and implant models are shown in Table 1.
The total load at the implant abutment is 11 N, 1 N at each node. The load application is cyclic in nature. The total cycles are 7200 in 3600 sec with frequency 2 cycles/sec as shown in Fig. 5. The investigating area for stress distribution pattern and displacement is the bone-implant interface, at the border between the dental implant, resilient material, cortical bone and oral mucosa as shown in Fig. 6.
We compare the effect of introducing different height polyoxymethylene resilient material abutment on the bone-implant interface with 3mm Titanium abutment. We compare the stress distribution pattern and displacement of Model 2, 3, and 4 with Model 1 in two-dimensional dynamic finite element analysis.
The finite element analysis executed by MARC/Mentat gives us the Von Mises stress pattern of the Model 1 and Model 2, 3 and 4. Von Mises stress was considered as it gives us unique value for all the six stress components.
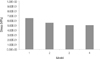
The stress distribution pattern of Model 1 and Model 2 is compared in Fig. 7. The bone-implant interface shows stress distribution pattern almost the same. There is slight reduction in stress magnitude as shown in Fig. 10.
Fig. 8 shows the stress pattern of Model 1 and Model 3. The stress distribution pattern similar is to Model 2. However, in this case the stress distribution pattern is narrower than Model 2 and stresses are slightly lower in magnitude in bone-implant interface as seen in Fig. 10.
The stress distribution of Model 1 and Model 4 is seen in Fig. 9. The stress distribution pattern is similar to Model 2 at the bone-implant interface. However, the stress distribution is slightly narrower than Model 3 and stresses are slightly lower in magnitude as seen in Fig. 10.
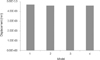
When a comparison was made between displacement at the bone-implant interface we conclude that the displacement pattern are almost equal as shown in Fig. 10.
The role of stress absorbing elements in implant system has been analyzed in various studies.10 The resilient material abutment can help in damping the height of peak stresses in dynamic loading and also in stress absorbing/ stress distributing the forces experienced by the dental implant system. The latter function depends upon the implant system and material properties.6 This experimental study uses finite element method to compare the stress distribution pattern and displacement on the bone-implant interface with the introduction of different lengths polyoxymethylene resilient material abutment, and 3 mm titanium abutment.
This study shows that polyoxymethylene resilient material abutment results in slight change in the stress magnitude in the bone-implant interface, which is insignificant. This may be due to polyoxymethylene resilient material abutment having Young's modulus (3,450 MPa) much lower than titanium (107,000 MPa). The reduction in Young's modulus leads to slight decrease in stress as compared to Model 1.
Our study is in accordance with a study by Geng et al.11, in which they concluded that there is no significant difference in stress when resilient material abutment was modeled in polyoxymethylene rather than titanium.
Changing the height of polyoxymethylene resilient material abutment leads to slight change in distribution of stress pattern, but it is also insignificant. This change can be attributed to the increased height of the polyoxymethylene resilient material abutment and lower Young's modulus of the polyoxymethylene resilient material abutment when compared to 3 mm titanium abutment.
The displacement pattern at the bone-implant interface shows, introduction of polyoxymethylene resilient material abutment leads to slight change in the displacement magnitude, which is inappreciable. Also changing the height of the polyoxymethylene resilient material abutment leads to inappreciable change in displacement magnitude when compared with 3 mm titanium abutment.
In this experimental study, we have introduced dynamic loading in finite element analysis. In oral cavity most of loading is dynamic in nature so evaluating the loading conditions in static loading leads to simplification of the complex biomechanical process taking place inside the oral cavity.
With the introduction of dynamic loading there is no significant change in the magnitude of the stress or the displacement. This may be attributed to the fact that there are no cumulative stresses transferred to the cortical bone which may show increase in stress magnitude with time. Hence, the magnitude of the stress and displacement remains the same in dynamic loading.
The introduction of lower Young's modulus polyoxymethylene resilient material abutment does not lead to significant change in the stress and displacement pattern as compared to titanium abutment in dynamic loading. Hence introduction of lower Young's modulus does not change the parameters significantly, in dynamic loading in relation of stress and displacement distribution.
There are limitations in this study as the bone is assumed to be homogeneous, isotropic, while the bone is non-homogeneous and anisotropic. The load applied is 11 N, as we were investigating the change in the stress distribution pattern and displacement, which can easily be accomplished by applying 1 N load for all the 11 nodes on the top of the abutment. The total time evaluated in this study was 3600 seconds, in order to ease computational load, as all non-linear finite element analysis takes more time than static finite element analysis to solve the mathematical model. The above mentioned applied load and conditions indicate that the stress results are not absolute but relative in nature. This is a qualitative analysis and not a quantitative analysis with 2 dimensional finite element method.
The marginal bone loss is due to one or combination of the following factors i.e. occlusal overload, microgap, and impact crest module.12 In this study we changed the height and young modulus of the abutment material as one of variations of occlusal overload in order to study its effects on marginal bone loss.
Within the limitation of this study we can conclude that changing the Young's modulus and height of the resilient material abutment does not significantly change the stress and displacement pattern at the bone-implant interface as compared to 3 mm titanium abutment. This leads us to conclude that the dental implant and bone interface is not influenced by the addition of lower Young's modulus resilient material abutment.
Clinically, the introduction of polyoxymethylene resilient abutment material does not cause any significant changes in stress and displacement pattern at bone-implant interface hence its introduction does not play any role in marginal bone loss (saucerization).
Figures and Tables
References
1. Kirsch A, Mentag PJ. The IMZ endosseous two phase implant system: a complete oral rehabilitation treatment concept. J Oral Implantol. 1986. 12:576–589.
2. Babbush CA, Kirsch A, Mentag PJ, Hill B. Intramobile cylinder (IMZ) two-stage osteointegrated implant system with the intramobile element (IME): part I. Its ratinale and procedure for use. Int J Oral Maxillofac Implants. 1987. 2:203–216.
3. Achour T, Merdji A, Bachir BB, Serier B, Djebbar N. Stress distribution in dental implant with elastomeric stress barrier. Mater Des. 2011. 32:282–290.
4. Holmes DC, Grigsby WR, Goel VK, Keller JC. Comparison of stress transmission in the IMZ implant system with polyoxymethylene or titanium intramobile element: a finite element stress analysis. Int J Oral Maxillofac Implants. 1992. 7:450–458.
5. McGlumphy EA, Campagni WV, Peterson LJ. A comparison of the stress transfer characteristics of a dental implant with a rigid or a resilient internal element. J Prosthet Dent. 1989. 62:586–593.
6. van Rossen IP, Braak LH, de Putter C, de Groot K. Stress-absorbing elements in dental implants. J Prosthet Dent. 1990. 64:198–205.
7. Sato M, Kuboki T, Itasaka M, Kondo Y, Yamashita A. Stres-stransfer characteristics of stress-absorbing elements in the IMZ implant system: Part 1. Stress around a freestanding single implant under static loading conditions. J Jpn Prosthodont Soc. 1992. 36:1102–1110.
8. Haganman CR, Holmes DC, Aquilino SA, Diaz-Arnold AM, Stanford CM. Deflection and stress distribution in three different IMZ abutment designs. J Prosthodont. 1997. 6:110–121.
9. Morton D, Stanford CM, Aquilino SA. Evaluation of resilient abutment components on measured strain using dynamic loading conditions. J Prosthet Dent. 1998. 80:46–51.
10. Skalak R. Biomechanical considerations in osseointegrated prostheses. J Prosthet Dent. 1983. 49:843–848.
11. Geng JP, Tan KB, Liu GR. Application of finite element analysis in implant dentistry: a review of the literature. J Prosthet Dent. 2001. 85:585–598.
12. Oh TJ, Yoon J, Misch CE, Wang HL. The causes of early implant bone loss: myth or science? J Periodontol. 2002. 73:322–333.




 PDF
PDF ePub
ePub Citation
Citation Print
Print











 XML Download
XML Download