Abstract
Large oro-facial defects result from cancer treatment consequences in serious functional as well as cosmetic deformities. Acceptable cosmetic results usually can be obtained with a facial prosthesis. However, retention of a large facial prosthesis can be challenging because of its size and weight. This article describes prosthetic rehabilitation of a 57-year-old man having a right lateral mid-facial defect with intraoral-extraoral combination prosthesis. A modified technique to fabricate a hollow substructure in heat-polymerizing polymethyl-methacrylate to support silicone facial prosthesis was illustrated. The resultant facial prosthesis was structurally durable and light in weight facilitating the retention with magnets satisfactorily. This technique is advantageous as there is no need to fabricate the whole prosthesis again in case of damage of the silicone layer because the outer silicone layer can be removed and re-packed on the substructure if the gypsum-mold is preserved.
Midfacial defects may result from congenital or developmental abnormalities, accidental trauma, or acquired disfigurements resulting from removal of tumors during maxillectomy surgery in the oral or nasal cavities. Midfacial defects occur in the horizontal plane of the middle third of the face including 2 main categories: midline and lateral defects. Midline defects refer to the complete or partial involvement of the nose, and/or upper lip, along with intraoral maxillary defects.1,2 A lateral defect may include complete or partial contents of the cheek and or orbit, and may include an intraoral defect of the maxilla.1-3 Acquired midfacial defects may affect speech, mastication, quality of life, psychology, and social behavior.4-7 Large defects that result from cancer treatment are rarely rehabilitated by surgical reconstruction alone: they usually require a facial prosthesis to restore function and appearance.8 In addition, an intraoral prosthesis such as an obturator is often needed to restore speech and deglutition. Fabrication of an extraoral facial prosthesis challenges the artistic ability of the prosthodontist. Retention of the prosthesis is also a difficult problem because of its size and weight. This clinical report describes the Prosthodontic rehabilitation of a dentate patient with a large midfacial (intraoral-extraoral) surgical defect. A modified technique to fabricate a hollow light-weight facial prosthesis is described.
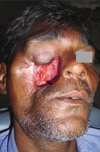
A 57-year-old man, visited to the Department of Ear-nose-throat in Government medical college and hospital, Nagpur (India) due to the extensive ulceration in right maxillary cheek mucosa and the swelling in the right maxillary buccal vestibule. Clinico-pathological examination revealed T4N3M0 squamous cell carcinoma of right maxilla and cheek mucosa. The patient was then operated for right orbital exenteration and right zygomatic resection along with resection of the right maxilla to eradicate all possible cancerous tissues. The patient received a postoperative course of total dose of 7200 cGy external beam radiation within a period of 7 weeks in fractionations (a fraction of 200 cGy/day for 5 days in a week). The patient was restricted to a liquid-diet through a naso-gastric tube for initial 3 months after surgery. A surgical-obturator was fabricated and tried intraorally after surgery, but due to extensive intraoral tissue resection (also communicated extraorally) complete prosthetic-closure of the defect was not achieved well. The patient was not able to control liquids in the oral cavity and hence refused to wear the obturator at initial healing phase. After 3 months of healing period, the patient was referred to the Department of Prosthodontics in Government Dental College and Hospital, Nagpur (India) for prosthetic rehabilitation where he was delivered an interim obturator (without teeth incorporated). Patient had utilized the interim obturator for last 6 months. After 9 months of surgery the patient was examined in the Department of Ear-nose-throat for healing of the defect and was referred to the Department of Prosthodontics for fabrication of a definitive Prosthesis. Extraoral and intraoral examination revealed complete healing of the surgical wound (Fig. 1). Mucosal quality over the remaining portion of the hard palate was normal and healthy. Periodontal condition of mandibular teeth and remaining maxillary teeth (on left side) were examined clinically as well as radiographically (with the help of Orthopantomogram) and observed to be in healthy status. Examination of Orthopantomogram revealed loss of floor of right orbit, right zygoma and right half of the maxilla along with the teeth. Condylar processes and glenoid fossae on both sides were found to be normal in shape in Orthopantomogram. Temporomandibular joint and the muscles of mastication were palpated on both sides to evaluate range of mandibular movements and found to be normal. Deglutition and speech intelligibility were drastically affected as the patient's tongue could not make effective functional contacts due to lack of anatomic (palatal, alveolar, dental) boundaries during deglutition and phonetics. Due to extensive size of the defect, radiation to the area, poor mucosal quality and minimal bony supporting structures; long-term prognosis of the implant-retained prosthesis was poor. Hence prosthetic rehabilitation was planned with a magnet retained intraoral-extraoral combination prosthesis (IECP) instead of an implant retained prosthesis. Fabrication of the IECP was planned in 2 sections. Intraoral obturator prosthesis was fabricated first followed by fabrication of an extraoral facial prosthesis.
Preliminary impressions of the normal mandibular arch as well as remaining maxillary arch along with the intraoral defect were made using high viscosity irreversible hydrocolloid (Dentalgin; Prime Dental Products, Mumbai, India). The impressions were poured in type III gypsum material (Kalstone; Kalabhai Karson, Mumbai, India). Maxillary custom impression tray was fabricated and adjusted for proper extensions. The tray was border molded with green stick impression compound (Pinnacle; Dental Products of India, Mumbai, India) including the palatal defect. The impression compound was relieved and a physiologic definitive impression was made using a medium viscosity polyvinyl-siloxane impression material (Reprosil; Dentsply DeTrey GmbH, Konstanz, Germany). Conventional prosthodontic protocols of boxing and pouring the impression with type III gypsum material were used to create a maxillary definitive cast. A 19 gauge hard, round, stainless steel orthodontic wire (KC Smith & Co, Monmouth, UK) was manipulated to make a fitted labial bow from the mesial aspect of the left central incisor to the distal aspect of left second molar. Though the surgical defect was completely healed chance of recurrence of the lesion was suspected hence a cast metal framework was not planned. A processed record base was fabricated from heat-polymerizing polymethyl-methacrylate (PMMA) (Trevalon; Dentsply, York, PA, USA) in conventional manner and adjusted with the aid of pressure indicating paste to allow complete seating.9 The definitive cast (used to fabricate the processed record base) was damaged on the lateral aspect during retrieval of the record base from the undercut areas. A sheet of baseplate wax (Modelling wax; Deepti Dental Products, Ratnagiri, Maharashtra, India) was adapted and contoured over the palatal defect portion of the processed record base to restore normal palatal contour and close the palatal portion of the defect intraorally. The adapted wax sheet was removed from the record base without distortion, flasked and processed in heat-polymerizing PMMA. The palatal piece was secured to the processed record base with auto-polymerizing PMMA and an occlusion rim was fabricated with baseplate wax. Jaw relation was recorded and transferred onto a semi-adjustable articulator with the help of face-bow. Teeth arrangement and wax contouring were carried out and the waxed-up prosthesis was tried in patient's mouth. After try-in the waxed-up obturator was processed in heat-polymerizing PMMA using conventional technique.9 After finishing and polishing the obturator, the gingival portion of the prosthesis was color modified with the auto-polymerizing clear PMMA (Cold cure-clear, Dental Products of India, Mumbai, India) mixed with acrylic-colors to match the normal melanin-pigmented gingiva of the contralateral side. The prosthesis was delivered to the patient and final occlusal refinement was completed (Fig. 2). The patient experienced improvement in speech intelligibility and deglutition after the placement of the maxillary obturator. A pair of Cobalt-Samarium magnets 30 mm in diameter and 2.5 mm in thickness (Jobmasters, Randallstown, MD, USA) was selected for mutual retention between intraoral and extraoral prostheses. One of the magnets was embedded in the superior-lateral aspect (portion which was exposed extraorally) of the obturator by using auto-polymerizing PMMA thus completing the intraoral section of the IECP (Fig. 3).
The patient was instructed to close in maximum intercuspal position with obturator placed in the mouth. The second magnet was placed over the obturator magnet. An irreversible hydrocolloid facial-moulage was made to record the facial defect along with surrounding normal extraoral structures and the extraorally exposed portion of the obturator with the second magnet placed over the obturator-magnet. A definitive cast was formed from type III gypsum. A single thickness baseplate wax was adapted over the extraoral defect area of the definitive cast. The wax sheet was flasked and processed in heat polymerized clear PMMA (Trevalon clear; Dentsply, York, PA, USA) using conventional technique.9 The inner PMMA framework was tried over the patient's extraoral defect by placing obturator and second magnet in position. An indentation for the second magnet was formed on the tissue surface of the framework. A cellophane paper (DPI, Mumbai, India) was placed in between the obturator magnet and the second magnet to act as a separating medium. Auto-polymerizing clear PMMA was mixed and placed in the indentation of the second magnet formed inside the tissue surface and the framework was seated over the defect area. After completion of polymerization, the second magnet was embedded in the framework (Fig. 4). An artificial stock-eye globe was selected to match size, shape and color of the normal left eye. The sclera was further color modified (from back side leaving front clear polished surface intact) with auto-polymerizing clear PMMA mixed with acrylic-colors to match the normal left eye sclera. The eye globe was positioned on the framework with reference to normal left eye and fixed with auto-polymerizing clear PMMA.
A sheet of baseplate wax was adapted over the inner framework in such a way that it would provide uniform thickness of the silicone for future facial contour over the defect area (Fig. 5). This was carried out by completely filling the wax on the inner framework and contouring the wax according to the facial contour, which was further cut back uniformly (4 mm in depth) from all over the facial surface. Petroleum jelly was applied over the cut back surface and a single thickness wax sheet was adapted to ensure uniform 3 mm of space for future overlying silicone layer. The adapted wax sheet was separated from the underlying wax surface without distortion and separately flasked for processing in heat-polymerizing PMMA. The underlying wax over the framework was removed by dewaxing procedure. The processed top layer framework was secured in position with inner framework using the auto-polymerizing PMMA thus completing a hollow PMMA substructure (Fig. 6).
The final portion of the facial prosthesis was sculpted with baseplate wax over the completed hollow PMMA substructure (Fig. 7). The wax sculpture was evaluated by positioning it on the patient's face. The wax sculpture of the prosthesis was invested in type IV gypsum material (Ultrarock; Kalabhai Karson, Mumbai, India) to form a mold for packing the silicone. Dewaxing was carried out in usual manner.9 The prosthesis was packed with a MDX4-4210-base silicone (Dow Corning Corp, Midland, MI, USA) and colored using intrinsic stains (KT-699, Silicone Coloring Kit; Factor II, Lakeside, AZ, USA) selected according to the patient's skin color. The silicone was heated for 2 hours at 90℃, deflasked, trimmed, cleaned, and bonded to the underlying framework with medical adhesive type A (Factor II) under vacuum as described by Lemon et al.10 Polyurethane lining was applied to the margins to increase the tear resistance of the marginal silicone.10,11 The prosthesis was trial fitted and extrinsically colored with trichlorethane, medical adhesive type A and oil pigments (Factor II) thus completing the fabrication of extraoral section of the IECP. The gypsum-mold was preserved for future re-packing in case of discoloration or damage of the overlying silicone layer.
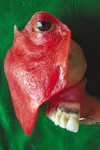
The patient was referred to an ophthalmologist for routine evaluation of the left eye. He was found to have hypermetropia. A spectacle frame was selected and the left glass of the spectacle was fabricated according to the specifications provided by the ophthalmologist to treat the hypermetropia of the left eye. During the prosthesis insertion appointment the maxillary obturator was placed intraorally and the facial prosthesis was positioned extraorally against the obturator magnet (Fig. 8). The patient was given hygiene instructions for cleaning the IECP. The patient attended recall visits every 4 to 5 months. During these visits, the obturator and facial prosthesis were thoroughly cleaned with a disinfectant (MD 520; Dürr Dental GmbH, Bietigheim-Bissingen, Germany). Two years after prosthesis insertion the prosthesis was still serviceable and the patient was pleased. The patient has been able to maintain 100% of his weight with a normal diet and nutritional supplements ingested orally. A cosmetic improvement, the ability to speak more intelligibly, improved deglutition and improved mastication was achieved for this patient with this IECP.
Large orofacial defects result in serious functional (impairment of speech, mastication, and deglutition) as well as cosmetic deformity. The cosmetic deformity often has a significant psychological impact upon the patient. Acceptable cosmetic results usually can be obtained with a facial prosthesis. However, retention of a large prosthesis can be challenging. With ingenuity and an understanding of the remaining anatomic structures, intraoral and extraoral prostheses that mutually retain one another can be constructed. Various methods of auxiliary retention for facial prostheses have been described in the literature; they include eyeglasses,12 extensions from the denture that engage tissue undercuts,12,13 magnets,12,14 adhesives,12 combinations of the above,12,13-15 and osseointegrated implants.12,13,16,17 Although osseointegrated implants may provide the most reliable prosthesis retention; additional surgeries, expenses, inadequate bone, and prior radiation to the area may contraindicate this type of treatment.18,19 The prosthetic rehabilitation of a patient with a combined intraoral-extraoral defect has been presented in this article. A 2-piece (intraoral obturator and facial) combination prosthesis was fabricated. Magnets provided mutual retention of the IECP. This was an esthetic option as there was sufficient space to utilize magnets without hindering the external appearance of the prosthesis.
Several authors have reported different problems that compromise the serviceability of facial prostheses made of a combination of PMMA and silicone.4,20 These include degradation of the silicone properties, delamination of silicone from the PMMA base, reduced marginal integrity of the facial prosthesis, resulting in open margins, and poor simulation of facial expressions due to the rigidity and heavy-weight of the PMMA base.4,20 Increased bulk of the PMMA framework was always a worry for the prosthodontists. There has been increased interest in using a fiber-reinforced composite as a dental and medical biomaterial for the fabrication of a facial prosthesis framework which would be light-in-weight.3 This requires more sophisticated techniques and expensive materials than PMMA. This article describes a technique to make a light-weight PMMA substructure by making it hollow. The light-weight facial prosthesis facilitates better retention with magnets. The problems of delamination of silicone from PMMA base can be easily overcome by bonding the processed silicone to an underlying substructure with medical adhesive type A under vacuum as described by Lemon et al.10 This technique is advantageous as there is no need to fabricate the whole prosthesis again in case of discoloration or damage of the silicone layer because the outer silicone layer can be removed and re-packed with the new silicone on the PMMA substructure if the mold is preserved.
Cast-metal framework was not planned and the Orthodontic wire clasps were used for retention of the intraoral prosthesis as recurrence of the lesion was suspected for initial 2-3 years. The cast-metal framework was avoided initially due to complex clinical as well as laboratory procedures. Though in this case report the cast metal framework was not utilized, it is mandatory to re-fabricate the intraoral prosthesis using cast metal framework once the cancer-recurrence is ruled out. The cast-metal framework improves retention, stability, support and bracing of the prosthesis and thus increases the longevity of both prosthesis as well as supporting tissues. Major disadvantage of 2-piece prosthesis is that the mobility of intraoral obturator can make facial prosthesis mobile especially during functions. We did not find clinically significant vertical mobility or sinking down of the prosthesis during functional movements due to light weight of the prosthesis and good extraoral bony support of the remaining orbital roof and zygoma. Durability of surface-coatings of the long-term magnets is a major concern; hence it is advised to use the magnets with strong surface coatings. Periodic recall appointments at the interval of 6 months are advisable for assessment of the prosthesis (retention, stability and support) and the supporting tissues.
Satisfactory functional and esthetic results are achievable in patients with a large lateral midfacial defect using a hollow PMMA substructure for silicone facial prosthesis. Retention of facial prosthesis can be satisfactorily achieved with the use of a pair of magnets provided the facial prosthesis is light in weight.
Figures and Tables
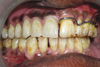 | Fig. 2Intraoral view of completed obturator prosthesis. Note gingival portion of prosthesis was color modified to match opposite side melanin-pigmented gingiva. |
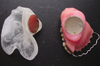 | Fig. 4Counter-magnet fixed inside the tissue surface of PMMA framework by maintaining correct relationship of the framework with the defect and the exposed portion of the obturator. |
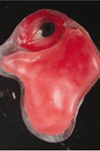 | Fig. 5Wax sheet contoured to allow 3 mm of uniform thickness of silicone for future normal facial contour. |
 | Fig. 6Separately processed top layer framework attached with underlying framework using auto-polymerizing PMMA. |
ACKNOWLEDGEMENTS
Author thanks Dr. Hirasankar Bhowmik (Lecturer, Department of Prosthodontics, Sinhagad Dental College and Hospital, Pune, Maharashtra, India) for his help in the treatment planning and Dr. Smita P. Patil (PG student, Department of Orthodontics, PMNM Dental College and Hospital, Bagalkot, Karnataka, India) for her help in the manuscript preparation.
References
1. Marunick MT, Harrison R, Beumer J 3rd. Prosthodontic rehabilitation of midfacial defects. J Prosthet Dent. 1985. 54:553–560.
2. Guttal SS, Patil NP, Shetye AD. Prosthetic rehabilitation of a midfacial defect resulting from lethal midline granuloma-a clinical report. J Oral Rehabil. 2006. 33:863–867.
3. Kurunmäki H, Kantola R, Hatamleh MM, Watts DC, Vallittu PK. A fiber-reinforced composite prosthesis restoring a lateral midfacial defect: a clinical report. J Prosthet Dent. 2008. 100:348–352.
4. Thomas KF. The art of clinical anaplastology. Techniques and materials guide for successful facial and somato prosthetic rehabilitation. 2006. 2nd Ed. London: S Thomas;16–22.
5. The glossary of prosthodontic terms. J Prosthet Dent. 2005. 94:10–92.
6. Schaff NG. Winkler S, editor. Maxillofacial prosthetics. Essentials of complete denture prosthodontics. 1994. St. Louis: Ishiyaku EuroAmerica;403–415.
7. Hecker DM, Wiens JP, Cowper TR, Eckert SE, Gitto CA, Jacob RF, Mahanna GK, Turner GE, Potts A, Logan H, Wiens RL. Can we assess quality of life in patients with head and neck cancer? A preliminary report from the American Academy of Maxillofacial Prosthetics. J Prosthet Dent. 2002. 88:344–351.
8. Brignoni R, Dominici JT. An intraoral-extraoral combination prosthesis using an intermediate framework and magnets: a clinical report. J Prosthet Dent. 2001. 85:7–11.
9. Morrow RM, Rudd KD, Rhoads JE. Dental laboratory procedures complete dentures. 1986. 2nd ed. St. Louis: Mosby;312–338.
10. Lemon JC, Martin JW, King GE. Modified technique for preparing a polyurethane lining for facial prostheses. J Prosthet Dent. 1992. 67:228–229.
11. Udagama A. Urethane-lined silicone facial prostheses. J Prosthet Dent. 1987. 58:351–354.
12. Thomas K. Prosthetic rehabilitation. 1994. London: Quintessence Publishing;93–103.
13. Beumer J III, Curtis TA, Marunick MT. Maxillofacial rehabilitation: prosthodontic and surgical considerations. 1996. St Louis: Ishiyaku EuroAmerica Inc;408–416.
14. Dumbrigue HB, Fyler A. Minimizing prosthesis movement in a midfacial defect: a clinical report. J Prosthet Dent. 1997. 78:341–345.
15. Verdonck HW, Peters R, Vish LL. Retention and stability problems in a patient with a large combined intra- and extraoral defect: a case report. J Facial Somato Prosthet. 1998. 4:123–127.
16. Menneking H, Klein M, Hell B, Bier J. Prosthetic restoration of nasal defects: Indications for two different osseointegrated implant systems. J Facial Somato Prosthet. 1998. 4:29–33.
17. Worthington P, Brånemark PI. Advanced osseointegration surgery: Applications in the maxillofacial region. 1992. Carol Stream, Ill: Quintessence;307–326.
18. Arcuri MR, LaVelle WE, Fyler E, Jons R. Prosthetic complications of extraoral implants. J Prosthet Dent. 1993. 69:289–292.
19. Roumanas E, Nishimura R, Beumer J III, Moy P, Weinlander M, Lorant J. Craniofacial defects and osseointegrated implants: Six-year follow-up report on the success rates of craniofacial implants at UCLA. Int J Oral Maxillofac implants. 1994. 9:579–585.
20. Taft RM, Cameron SM, Knudson RC, Runyan DA. The effect of primers and surface characteristics on the adhesion-in-peel force of silicone elastomers bonded to resin materials. J Prosthet Dent. 1996. 76:515–518.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download