INTRODUCTION
MATERIAL AND METHODS
Fabrication of titanium substrata
Contact angle determination
Cell culture
Bromodeoxyuridine cell proliferation assay
Alkaline phosphatase activity test
Statistical analysis
RESULTS
Contact angle determination
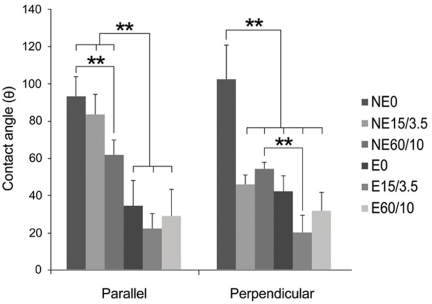 | Fig. 2Multiple-comparison result of the contact angle determination on titanium substrata with various surface topographies measured in directions parallel with and perpendicular to the microgrooves. Statistical significances were tested among NE0, NE15/3.5, NE60/10, E0, E15/3.5, and E60/10 using one-way ANOVA (n = 3). **: significant difference (P <.01). |
Table I
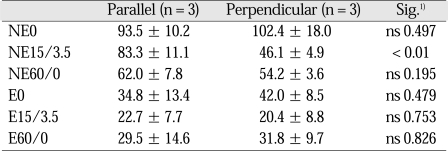
1) Statistical significances were tested by Student t-test with equal variances assumed. ns: non-significant.
See table I for nomenclature.
Bromodeoxyuridine cell proliferation assay
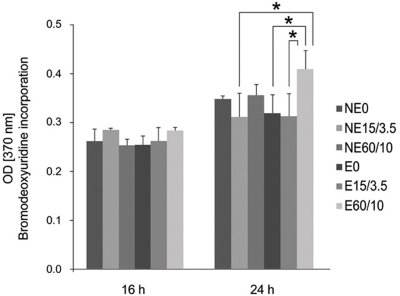 | Fig. 3Multiple-comparison result of the cell proliferation of MC3T3 mouse preosteoblasts on titanium substrata with various surface topographies after 16 and 24 h of culture using bromodeoxyuridine assay. Statistical significances were tested among NE0, NE15/3.5, NE60/10, E0, E15/3.5, and E60/10 using one-way ANOVA (n = 3). *: significant difference (P <.05). |
Alkaline phosphatase activity test
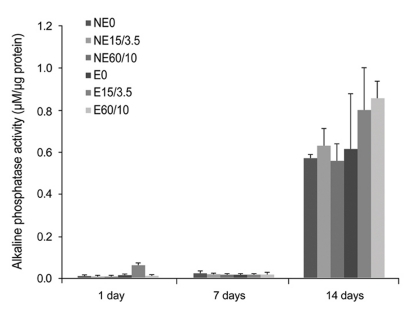 | Fig. 4Multiple-comparison result of the alkaline phosphatase activity test of MC3T3 mouse preosteoblasts on titanium substrata with various surface topographies after 1, 7 and 14 days of osteogenic culture. Statistical significances were tested among NE0, NE15/3.5, NE60/10, E0, E15/3.5, and E60/10 using one-way ANOVA (n = 3). The result showed no significant differences in the osteoblastic differentiation between and within all groups (P < .05). |
Correlation and regression analyses
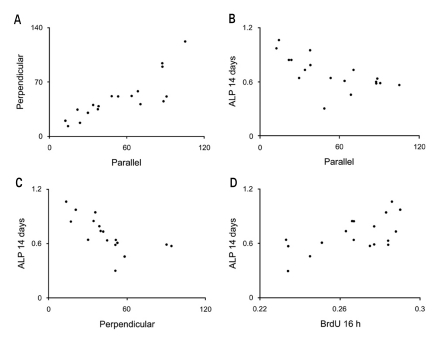 | Fig. 5Scatter-plot results from the Pearson's correlation analysis. Correlations of the data and results between A Parellel and Perpendicular, B Parellel and ALP 14 days, C Perpendicular and ALP 14 days and D BrdU 16 h and ALP 14 days are presented. Significant correlations were present A, B, C and D (P < .01). See table II for nomenclature and the overall results. |
Table II
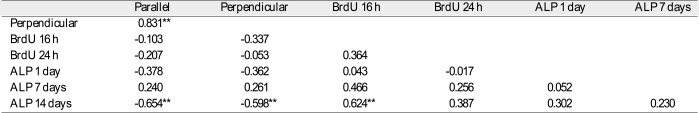
**: Correlation is significant at the 0.01 level (2-tailed). N = 18. Parallel and Perpendicular: the result from the contact angle analysis in the directions parallel with and perpendicular to the microgrooves. BrdU αh: the result from the bromodeoxyuridine assay of MC3T3 mouse preosteoblasts at αh incubation. ALP βday(s): the result from the alkaline phosphatase activity test of MC3T3 mouse preosteoblasts after βday(s) of osteogenic culture.
Table III
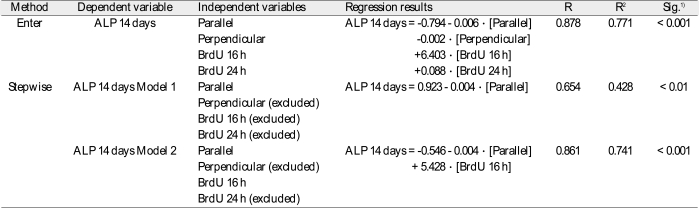
1) Significances were tested by analysis of variance. R: coefficient of multiple correlations. R2: coefficient of determination. N = 18. See table 2 for nomenclature.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download