Abstract
PURPOSE
One of the major keys to achieve successful osseointegration of the implant is its surface properties. The aim of this study was to investigate the bone response to dental implants with different surface characteristics using the rabbit tibia model. Tricalcium phosphate (TCP) coated, anodic oxidized and turned (control) surfaces were compared.
MATERIALS AND METHODS
Seventy two implants were placed in the tibia of eighteen rabbits. Nine rabbits were sacrificed at 3 weeks of healing and the remaining nine were sacrificed at 6 weeks of healing. The bone-to-implant contact (BIC) and the bone volume density (BVD) were assessed by light microscope after 3 and 6 weeks of healing.
RESULTS
Statistical analysis showed that no significant differences in the BIC and BVD were observed between the different implant surfaces and the control group at 3 weeks and 6 weeks of healing. Data also suggested that the BVD of all the surfaces showed significant difference at 3 and 6 weeks.
CONCLUSION
The present study has showed that osseointegration occurred in all investigated types of surface-treated implants. In the current study all of the threads of the implants were observed to calculate BIC and BVD values (instead of choosing some of the threads from the bone cortex for example), which didn't make BIC or BVD percentage values better than in the control group, therefore the clinical relevance of these results remains to be shown.
As a result of the increasing number of implant placement in the clinical field, it is possible to observe the advent of several types of implants with different materials, designs and surface topography. Albrektsson and Brånemark1 have listed factors that assure osseointegration such as: implant biocompatibility, fixture design, surface characteristics, surgical techniques, state of host, biomechanical status and time. Among these factors, the surface morphology of dental implants has received increasing attention in recent years.
Currently, commercially pure titanium (cpTi) is the material of choice for dental implants due to its biocompatibility to bone, high resistance to corrosion and light weight compared to other materials. In addition, cpTi can be easily prepared and modified into any desired form without difficulties.2,3
Regarding the knowledge that above mentioned implant features significantly influence the formation of bone at implant surfaces, several methods were introduced to alter the surface topography4: coating by plasma spraying, abrasion, blasting, blasting and etching, anodizing, cold working, sintering, magnetron sputtering, electropolishing and laser preparation.5
Osseointegration is obtained by cellular events that lead to bone formation at the alloplastic surface of the implant6,7 and Larsson et al. reported that the functional activity of the cells close to the implant surface is highly influenced by the chemical, physical, mechanical and topographic properties of the surface.8
One of the methods to improve the cellular reaction at the implant surface is to control the roughness of the implant surface.9 Osteoblasts initially attach to the rough surfaces of Ti and further the roughness showed to have an effect on osteoblast proliferation and differentiation.10,11 On the contrary, fibroblasts showed low affinity to rough surfaces and failed to adhere to them.10 In addition, alkaline-phosphatase activity was increased by rough surfaces.11,12
Coating implants with calcium phosphate ceramics are known as an achievement way for rapid fixation and strong bonding between the bone and the implant.13,14 Bioactive coating of implants was found to establish a chemical bond along the bone/coating interface (bonding osseogenesis) including ion exchange with the host tissue.15,16
Another approach that enhances the bone response is to increase the thickness of the titanium oxide at the implant surface.17,18 Anodizing oxidation is an electrochemical process that increases the titanium oxide thickness and roughness.9
The purpose of this study was to analyze and compare the bone responses to 3 different types of implant surfaces: tricalcium phosphate (TCP) - coated, anodized, and turned (machined) surface implants using the rabbit tibia model.
Seventy-two turned screw-shaped implants (3.75 mm in diameter, 7.0 mm in length) (Osstem Implant, Pusan, Korea) were made from commercially pure titanium (grade IV). Twenty-four implants were not altered and remained as the control group while the other forty-eight implants' surfaces were treated according to two different techniques.
Among the forty-eight implants, twenty-four were coated with TCP. The implants were grit-blasted with 20 µm TCP powder for 11 - 14 seconds and then the prepared TCP sol was coated onto it by dip spin coating at 8000 rpm for 1 minute. The TCP-coated implants were then dried at 70℃ and heat treated at 600℃ for 6 hours in high vacuum furnace.
The remaining twenty-four implants were treated with anodic oxidation. This process was carried out at 300 V for 22 minutes at 10℃ in an electrolyte solution which consisted of 0.25M H2SO4 and 0.25M H3PO4. The current density was 70 A/m2 and the thickness of the oxide layer was 10 µm.
In order to conduct the in vivo experiment, this study had to be approved by the Animal Research Committee of Seoul National University (approved number: SNU-081004-4) and all experiments were done in accordance with the Institute of Laboratory Animal Resources guidelines of Seoul National University. Eighteen New Zealand white male rabbits, weighing 2.5 - 3.5 kg, were used in this study.
Prior to the surgical step, the proximal tibia area's skin was shaved and washed with betadine and pre-operative antibiotic. After that, 0.12 g IM kanamycin was administered prophylactically. Following conventional (28.8 mg/kg ketamine and 11.7 mg/kg xylazine) and local anesthesia (1.8 ml of 2% lidocaine), surgical site preparation and drilling for implant placement were conducted as described in a previous study.3 After skin incision was made, the muscles of the proximal aspect of the tibia were dissected to elevate the periosteum. The implant hole drilling was carried under low rotational speed and profuse saline irrigation. The drills were used according to their order of increasing diameter, and no countersink preparation was done and finally the holes were tapped with a 3.3 mm tap. Each rabbit received 4 implants, 2 of which were installed in each tibia. The position of the implants in the tibia was randomly assigned. After the top of the implant was covered by cover screw, the periosteum and the fascia were sutured with chromic gut and the skin was sutured with silk. The rabbits recovered without complications and received 0.06 mg kanamycin IM per day for 3 days post-operatively.
After 3 and 6 weeks of healing, the rabbits were anesthetized and sacrificed with an intravenous administration of potassium chloride (KCl). Nine rabbits were sacrificed at 3 weeks of healing and the remaining nine were sacrificed at 6 weeks of healing. Each set of implants was surgically removed en bloc with an adjacent bone collar and immediately fixed in 4% neutral formaldehyde. Then, specimens were embedded in light-curing resin (Technovit 7200 VLC, Kultzer, Wehrheim, Germany). Following a method described by Donath et al,19 undecalcified ground sections were cut and prepared using the Exakt® system (Exakt Apparatebau, Norderstedt, Germany). The specimens were ground to an approximate thickness of 30 µm and stained with hematoxilin and eosin (HE-staining). An IBM personal computer connected to an Olympus BX microscope (Olympus, Tokyo, Japan) and image analysis software (Kappa PS30C Imagebase, Kappa Opto-electronics GmbH, Gleichen, Germany) was used to calculate the percentage of bone-to-implant contact (BIC) and bone volume density (BVD). All light microscopic calculations were done with a ×10 objective and ×10 eyepieces. The percentage of BIC in all of the threads at the bone cortex and intramedullary area was calculated.
As for the obtained results, one way analysis of variance (ANOVA) and Tukey HSD post hoc test were conducted to calculate possible statistical significances among the BIC and BVD of the investigated surfaces. The statistical significance of the differences in the percentage of BIC and BVD between 3 week samples and 6 week samples was assessed by independent t-test.
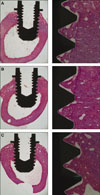
According to the light microscopic view, no inflammatory responses were observed in any specimens. It was also possible to observe a great amount of cancellous bone in contact with the dental implants at the marrow area. Regarding the cortical bone, it grew very little and didn't reach the implant surface at 3 weeks as opposed to the cortical bone at 6 weeks where it matured enough and anchored to the implant surface (Fig. 1, 2).
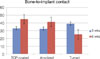
As for the BIC percentage of the specimens at 3 weeks, TCP-coated implants showed a percentage of 32.97 ± 30.57 while anodized implants showed a percentage of 32.54 ± 31.97 and turned implants 39.08 ± 33.13. At 6 weeks, TCP-coated implants showed a BIC percentage of 44.92 ± 31.86 while anodized implants showed a percentage of 41.42 ± 27.74 and turned implants 25.19 ± 24.66 (Table 1, Fig. 3).
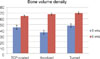
As a result of the BVD analysis, at 3 weeks TCP-coated implants showed a BV percentage of 45.13 ± 24.97 while anodized implants showed a percentage 36.40 ± 26.92 and turned implants 48.08 ± 31.09. At 6 weeks, TCP-coated implants showed a BVD percentage of 64.53 ± 27.60 while anodized implants showed a percentage of 68.19 ± 18.24 and turned implants 70.19 ± 13.09 (Table 2, Fig. 4).
Statistical analysis showed that no significant differences in the BIC and BVD were observed between the different implant surfaces and the control group at 3 weeks and 6 weeks of healing (P > .05). It was also possible to infer that BVD of all the surfaces (TCP-coated, anodized, and turned) showed statistically significant difference at 3 and 6 weeks.
In the current study, implants with different surface properties and the bone response to these implants were histomorphometrically analyzed in rabbits. Even though it is considered as a destructive method, histomorphometric measurement is a representative test in studying the nature of the implant-tissue surface and has been used by several authors to evaluate the bone-implant interface.20
A histological evaluation of the specimens in this study showed that osseointegration was achieved for all types of implants after a healing period of 3 and 6 weeks.
According to Larsson et al.,8,18,21 treatment of surface by changing the oxide thickness of titanium implants from an electropolished level to thick oxide layers formed by anodization provides polycrystalline metal surface with a crystalline oxide layer (porous regions on a nanometer level) and these implants show slightly improved response in bone, particularly in the first weeks after implantation.17 The anodic oxidation process used in this study was one of the several techniques available to produce adequate anodized surfaces.9
Tricalcium phosphate is applied to Ti implants in order to obtain rapid formation of new bone in contact with the implant at an early stage after implantation.22-24 Calcium phosphate coating has proved to be beneficial for the anchorage of metal implants in bone tissue25 and Lee et al. agreed on the proving, that TCP coating revealed to have excellent histological performance stimulating osteoconduction and osseointegration.26 Plasma spraying is the most widely used technique for biomedical application but does not allow to produce a uniform surface coatings thinner than about 40 µm.15 It must be said that many authors doubt the performance of plasma-sprayed coatings because of common cohesion failure at the coating/implant interface.27
Contrary to the hypothesis that treatment of implant surfaces would result in higher values of BIC and BVD for those, statistical analysis showed no significant differences in the BIC and BVD between the different implant surfaces and the control group at 3 and 6 weeks of healing. Yeo et al. investigated 3 surface-modified implants in their study and concluded that surface modification showed more favorable bone response than the control group which consisted of turned implants without surface treatment.9
It might be inferred that in the present study, surface-treated implants didn't show a better bone response to the implant surface due to the differences in observed areas and healing time until the sacrifice of animals. Yeo et al. analyzed the percentage of BIC in only four consecutive threads from the bone cortex.9 Previous studies have shown that in the rabbit tibia model, the implant is placed in contact almost exclusively with the cortical bone and that the bone formation is divided into 2 general steps: the first one occurring around the cortical portion of the screw (proximal 1 to 2 threads) and the second occurring around the intramedullary portion of the implants (the distal 3 to 4 threads).28,29
Schopper et al. reported that very high and uniform BIC values were present at implant portions placed into cortical bone, while implants portions placed into cancellous bone showed less high and less uniform BIC values. The authors also reported that a significant difference was present between cortical bone BIC and cancellous bone BIC values within the same implant, which is in accordance with the results of this current study in which an improved bone response was not observed due to the inclusion of less uniform cancellous bone BIC values in the total BIC value of the implants.24
However, the BIC values of cancellous bone should be included in the calculation of the implant's BIC value considering that the bone apposition to cancellous implant portions was highly correlated with total bone apposition. A good cancellous osseointegration must therefore be considered a major factor for implant success and bioactive coatings may provide benefit when predictable osseointegration is desired in bone of less quantity.30
It was also possible to infer through statistical analysis that the BVD of all the surfaces (TCP-coated, anodized and turned) showed significant difference at 3 and 6 weeks. Schliephake et al.31 also reported that the mean peri-implant BVD values increased significantly from 1 to 3 months in all analyzed implant groups.
There were some limitations in our study. The implants that were placed in the rabbit tibia were not loaded, which is a different situation from that of the implants placed in human jaws. Small sample size was also one of the limitations, especially regarding the BIC and BVD measurements. Studies with larger sample size are needed although there are strict limitations for animal studies.
The present study showed that osseointegration occurred in all investigated types of surface-treated implants. Due to the implant area observed for this study (all of the threads), surface-treated implants didn't make BIC or BVD percentage values better than in the control group, therefore the clinical relevance of these results remains to be shown.
Figures and Tables
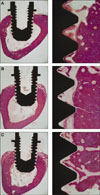 | Fig. 1Histologic view at 2 magnifications (×12.5, ×100) of (A) TCP-coated, (B) anodized, (C) turned implants after three weeks of healing. |
References
1. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981. 52:155–170.
2. Kasemo B, Lausmaa J. Surface science aspects on inorganic biomaterials. CRC Crit Rev Clin Neurobiol. 1986. 4:335–380.
3. Kim YH, Koak JY, Chang IT, Wennerberg A, Heo SJ. A histomorphometric analysis of the effects of various surface treatment methods on osseointegration. Int J Oral Maxillofac Implants. 2003. 18:349–356.
4. Pilliar RM. Overview of surface variability of metallic endosseous dental implants: textured and porous surface-structured designs. Implant Dent. 1998. 7:305–314.
5. Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000. 84:522–534.
6. Masuda T, Yliheikkilä PK, Felton DA, Cooper LF. Generalizations regarding the process and phenomenon of osseointegration. Part I. In vivo studies. Int J Oral Maxillofac Implants. 1998. 13:17–29.
7. Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD, Boyan BD. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996. 32:55–63.
8. Larsson C, Thomsen P, Lausmaa J, Rodahl M, Kasemo B, Ericson LE. Bone response to surface modified titanium implants: studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials. 1994. 15:1062–1074.
9. Yeo IS, Han JS, Yang JH. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J Biomed Mater Res B Appl Biomater. 2008. 87:303–311.
10. Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992. 7:302–310.
11. Schwartz Z, Martin JY, Dean DD, Simpson J, Cochran DL, Boyan BD. Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. J Biomed Mater Res. 1996. 30:145–155.
12. Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995. 29:389–401.
13. Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001. 58:570–592.
14. Park EK, Lee YE, Choi JY, Oh SH, Shin HI, Kim KH, Kim SY, Kim S. Cellular biocompatibility and stimulatory effects of calcium metaphosphate on osteoblastic differentiation of human bone marrow-derived stromal cells. Biomaterials. 2004. 25:3403–3411.
15. Fini M, Cigada A, Rondelli G, Chiesa R, Giardino R, Giavaresi G, Nicoli Aldini N, Torricelli P, Vicentini B. In vitro and in vivo behaviour of Ca- and P-enriched anodized titanium. Biomaterials. 1999. 20:1587–1594.
16. Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants. 2000. 15:675–690.
17. Ellingsen JE. Surface configurations of dental implants. Periodontol 2000. 1998. 17:36–46.
18. Larsson C, Thomsen P, Aronsson BO, Rodahl M, Lausmaa J, Kasemo B, Ericson LE. Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996. 17:605–616.
19. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982. 11:318–326.
20. Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998. 11:491–501.
21. Larsson C, Emanuelsson L, Thomsen P, Ericson LE, Aronsson BO, Kasemo B, Lausmaa J. Bone response to surface modified titanium implants - studies on the tissue response after 1 year to machined and electropolished implants with different oxide thicknesses. J Mater Sci Mater Med. 1997. 8:721–729.
22. Ducheyne P, Beight J, Cuckler J, Evans B, Radin S. Effect of calcium phosphate coating characteristics on early post-operative bone tissue ingrowth. Biomaterials. 1990. 11:531–540.
23. Chae JC, Collier JP, Mayor MB, Surprenant VA, Dauphinais LA. Enhanced ingrowth of porous-coated CoCr implants plasma-sprayed with tricalcium phosphate. J Biomed Mater Res. 1992. 26:93–102.
24. Schopper C, Moser D, Goriwoda W, Ziya-Ghazvini F, Spassova E, Lagogiannis G, Auterith A, Ewers R. The effect of three different calcium phosphate implant coatings on bone deposition and coating resorption: a long-term histological study in sheep. Clin Oral Implants Res. 2005. 16:357–368.
25. Clemens JA, Klein CP, Sakkers RJ, Dhert WJ, de Groot K, Rozing PM. Healing of gaps around calcium phosphate-coated implants in trabecular bone of the goat. J Biomed Mater Res. 1997. 36:55–64.
26. Lee TM, Wang BC, Yang YC, Chang E, Yang CY. Comparison of plasma-sprayed hydroxyapatite coatings and hydroxyapatite/tricalcium phosphate composite coatings: in vivo study. J Biomed Mater Res. 2001. 55:360–367.
27. Geesink RS, Groot KD, Klein CP. Bonding of bone to apatite-coated implants. J Bone Joint Surg Br. 1988. 70:17–22.
28. Sennerby L, Thomsen P, Ericson LE. Early tissue response to titanium implants inserted in rabbit cortical bone. part 1. Light microscopic observations. J Mater Sci Mater Med. 1993. 4:240–250.
29. Sennerby L, Thomsen P, Ericson LE. A morphometric and biomechanic comparison of titanium implants inserted in rabbit cortical and cancellous bone. Int J Oral Maxillofac Implants. 1992. 7:62–71.
30. Biesbrock AR, Edgerton M. Evaluation of the clinical predictability of hydroxyapatite-coated endosseous dental implants: a review of the literature. Int J Oral Maxillofac Implants. 1995. 10:712–720.
31. Schliephake H, Scharnweber D, Roesseler S, Dard M, Sewing A, Aref A. Biomimetic calcium phosphate composite coating of dental implants. Int J Oral Maxillofac Implants. 2006. 21:738–746.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download