This article has been
cited by other articles in ScienceCentral.
Abstract
PURPOSE
This research evaluated clinical outcomes of two types of hydroxyapatite (HA)-coated implants: OT (Osstem TS III-HA, Osstem implant Co., Busan, Korea) and ZM (Zimmer TSV-HA, Zimmer dental, Carlsbad, USA).
MATERIALS AND METHODS
The research was conducted on 303 implants (89 of OT, 214 of ZM), which were placed from January 16, 2010 to December 20, 2012. The prognosis was evaluated in terms of success rates, survival rates, annual marginal bone loss, and implant stability quotients (ISQ). The samples were classified into immediate, early, conventional, and delayed groups according to the loading time.
RESULTS
Overall, there were no significant differences between OT and ZM in success rates, survival rates, and annual marginal bone loss, except for the result of secondary stability. OT showed 77.83 ± 8.23 ISQ, which was marginally higher than 76.09 ± 6.90 ISQ of ZM (P<.05). In terms of healing periods, only immediate loading showed statistically significant differences (P<.05). Differences between OT and ZM were observed in terms of two indices, the annual marginal bone loss (0.17 ± 0.58 mm/year < 0.45 ± 0.80 mm/year) and secondary stability (84.36 ± 3.80 ISQ > 82.48 ± 3.69 ISQ) (P<.05). OT and ZM did not have any statistically significant differences in early, conventional, and delayed loading (P>.05).
CONCLUSION
OT (97.75%) and ZM (98.50%) showed relatively good outcomes in terms of survival rates. In general, OT and ZM did not show statistically significant differences in most indices (P>.05), although OT performed marginally better than ZM in the immediate loading and 1-stage surgery (P<.05).
Go to :

Keywords: Dental implant, Hydroxyapatite coating, Outcome
INTRODUCTION
Since the introduction of dental implant treatments in 1981 by Brånemark, efforts to achieve more rapid and stable osseointegration have continued.
1 Researchers have taken efforts to improve osseointegration and osteogenesis by coating the implant surface with hydroxyapatite, a major component of bone.
2 In particular, with demands for shortening the time of implant treatment and simplification of surgical procedures, especially when dealing with patients with poor bone quality, hydroxyapatite coating received much attention as a solution to address these issues.
34 However, early efforts were plagued by vulnerability to periimplantitis, hydroxyapatite dissolving after surgery, and failed interfacial adhesion between implant and hydroxyapatite. This led to the disappearance of hydroxyapatite from the market.
567 Subsequently, attention was focused on hydroxyapatite again owing to improved interfacial adhesion between implant and hydroxyapatite and advancements made in processes that allowed a more uniform coating. Examples of such advancements include ion-sputtering, process of thermal composition, and thermal plasma coating.
891011 Each implant manufacturer has its own proprietary coating process. A good example of this is MP-1, which is a thermal plasma coating technology applied to ZM (Zimmer TSV-HA, Zimmer Dental, Carlsbad, CA, USA) implants. The Zimmer TSV-HA used in this study achieved a 97% success rate because it increased the HA crystallization rate to 97% with the application of a plasma coating over the HA and a special MP-1 process using a compressed hydration heat treatment.
1213 Other manufacturers also produced several thermal plasma coating implants such as OT (Osstem TS III-HA, Osstem Implant Co., Busan, Korea). To improve osseointegration, it is important to increase the crystalline hydroxyapatite content while maintaining the strength of the implant itself. Consequently, manufacturers of hydroxyapatite-coated implants are developing their products with an emphasis on this aspect. OT was treated by the hybrid-type coating with HA and RBM, and the HA crystallinity was higher than 98%. Two millimeters of the upper fixture was RBM surface, and the rest was HA surface to improve the bone response in the cancellous bone, to restrict deposits of bacteria, and to lower marginal bone loss. The Ca/P ratio of the HA surface used in the above experiments was 1.76.
The present study conducted a comparative analysis on the prognosis between two thermal plasma hydroxyapatite-coated implants OT and ZM through a retrospective follow-up study for identifying the mid-term clinical outcomes (maximum 4.52 years, average 2.63 years) of OT, as only few studies have assessed the long-term clinical outcomes. Since the present study examined several implants (n = 303), it also reported on the results that were found for each implant under various conditions such as duration of treatment and surgical technique. The present study conducted a multi-dimensional analysis using success rate, annual marginal bone loss (aMBL), and implant stability quotient (ISQ) as prognostic indices, in addition to survival rates that previous studies primarily examined.
Go to :

MATERIALS AND METHODS
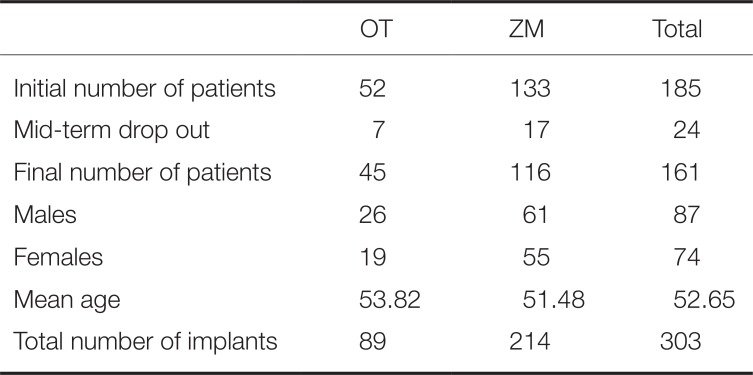
Between January 2010 and December 2012, 348 hydroxyapatite-coated implants were placed in 185 patients at Section of Dentistry, Seoul National University Bundang Hospital (SNUBH). Among them, 303 implants (89 OT and 214 ZM) that met criteria of the retrospective study were included in the study. The experimental group consisted of 161 patients (87 men and 74 women) with a mean age of 52.65 years (
Table 1).
Table 1
Number of patients and implants
|
OT |
ZM |
Total |
|
Initial number of patients |
52 |
133 |
185 |
|
Mid-term drop out |
7 |
17 |
24 |
|
Final number of patients |
45 |
116 |
161 |
|
Males |
26 |
61 |
87 |
|
Females |
19 |
55 |
74 |
|
Mean age |
53.82 |
51.48 |
52.65 |
|
Total number of implants |
89 |
214 |
303 |

All implant placement procedures were performed by a single oral maxillofacial surgeon, while all prosthetic treatments were performed by a single prosthodontist. All procedures were performed according to the manufacturer's protocol. The present study was conducted under the approval of Institutional Review Board at SNUBH (IRB No: B-1503-290-104). All participants received sufficient explanation on the details of the clinical trial and signed a consent form.
Inclusion criteria of this study were as follows: 1) patients who were 18 years or older 2) patients with manageable systemic disease 3) patients who had undergone minor guided bone regeneration and/or sinus lifting. Exclusion criteria were as follows: 1) patients who withdrew during the middle of the study 2) patients with insufficient medical records and/or radiologic examination records 3) patients who received prosthetic treatment at another hospital after fixture placement.
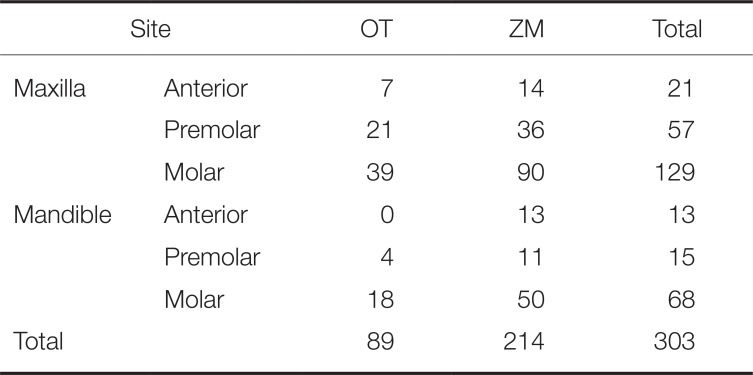
The implantation sites were as follows: 207 on the maxilla and 96 on the mandible and 34 on the anterior region, 72 on the premolar region, and 197 on the molar region. The highest number of implants was placed on maxillary molar region (129) (
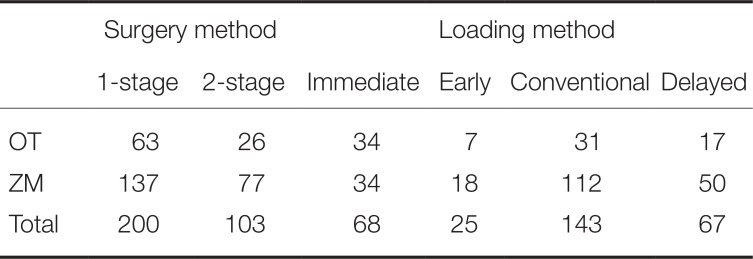
Table 2). Placement procedures consisted of two hundred (200) 1-stage surgeries and one hundred and three (103) 2-stage surgeries. The second surgery or impression taking was performed 2 – 3 months and 4 – 6 months after 1
st implant placement surgery in mandible and in maxilla, respectively. With respect to classification according to loading types, conventional loading was the most common with 143, followed in order by immediate loading with 68, delayed loading with 67, and early loading with 25 (
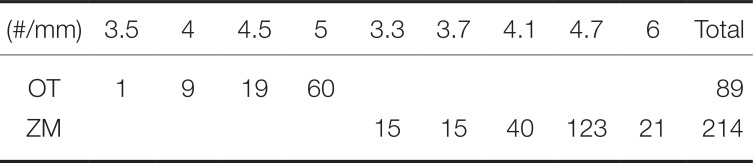
Table 3). Regarding the diameter of implants placed, 5-mm implant was the most common with 60 among OT, while 4.7-mm implant was the most common with 123 among ZM (
Table 4). In both groups, 10-mm implant was the most common (OT with 64, ZM with 144) length (
Table 5).
Table 2
Number of implants by placement site
|
Site |
OT |
ZM |
Total |
|
Maxilla |
Anterior |
7 |
14 |
21 |
|
Premolar |
21 |
36 |
57 |
|
Molar |
39 |
90 |
129 |
|
Mandible |
Anterior |
0 |
13 |
13 |
|
Premolar |
4 |
11 |
15 |
|
Molar |
18 |
50 |
68 |
|
Total |
89 |
214 |
303 |

Table 3
Number of implants by surgical and loading methods
|
Surgery method |
Loading method |
|
1-stage |
2-stage |
Immediate |
Early |
Conventional |
Delayed |
|
OT |
63 |
26 |
34 |
7 |
31 |
17 |
|
ZM |
137 |
77 |
34 |
18 |
112 |
50 |
|
Total |
200 |
103 |
68 |
25 |
143 |
67 |

Table 4
Number of implants by diameter
|
(#/mm) |
3.5 |
4 |
4.5 |
5 |
3.3 |
3.7 |
4.1 |
4.7 |
6 |
Total |
|
OT |
1 |
9 |
19 |
60 |
|
|
|
|
|
89 |
|
ZM |
|
|
|
|
15 |
15 |
40 |
123 |
21 |
214 |

Table 5
Number of implants by length
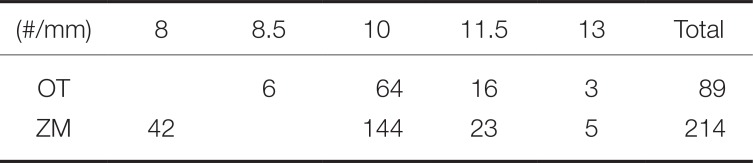
|
(#/mm) |
8 |
8.5 |
10 |
11.5 |
13 |
Total |
|
OT |
|
6 |
64 |
16 |
3 |
89 |
|
ZM |
42 |
|
144 |
23 |
5 |
214 |

The medical records and radiographs of the participants were retrospectively analyzed. Marginal bone loss (MBL), ISQ, success rate, and survival rate were investigated as outcome indices. In addition, comparisons were made according to the duration of healing and surgical method. The duration of healing refers to the time until prosthetic loading is exerted on the fixture, which was classified as immediate, early, conventional, and delayed loading. According to 2002 Barcelona consensus, immediate, early, conventional, and delayed loading was established as within 48 hours, 48 hours to ≤ 3 months, > 3 months to ≤ 6 months, and > 6 months, respectively.
14 Surgical method was classified as either 1-stage or 2-stage depending on whether a secondary surgery was required.
Periapical radiographs were acquired during regular follow-up via paralleling technique using RVG 6200 system (Carestream dental, Stuttgart, Germany). Picture archiving and communication system (PACS) used was Infinitt Marosis (Marotech, Seoul, Korea), and this was used to adjust the brightness and contrast of the radiographs to a set level. Marginal bone resorption was analyzed by 2 radiographs, one taken after implant placement and the other from the final examination dividing the changes in the height of marginal bone around the implant by imaging period. On Infinitt software, the height at the highest point of the marginal bone was measured relative to the top of the fixtureabutment connection. For error correction, the value from the software and actual fixture length were multiplied after establishing a proportional expression. Measurements were taken from two points, mesial and distal, and the means of values measured to two decimal places were used. Marginal bone height was analyzed by substituting the fixture height recorded at the time of implant placement into the equation shown in
figure 1. Using the method shown below, MBL (in mm) was derived by subtracting the final marginal bone level from the marginal bone level after the prosthesis was attached (
Fig. 1 and
Fig. 2). Since each case had different time frame on MBL measurement, it was necessary to compensate for such differences. The period from the first measurement date to the final measurement date was recorded in unit of days and 365 days was used as one year to divide the calculated values. This was expressed as aMBL and was compared accordingly. All values were obtained using Excel (Microsoft, Redmond, WA, USA) by setting up the aforementioned function.
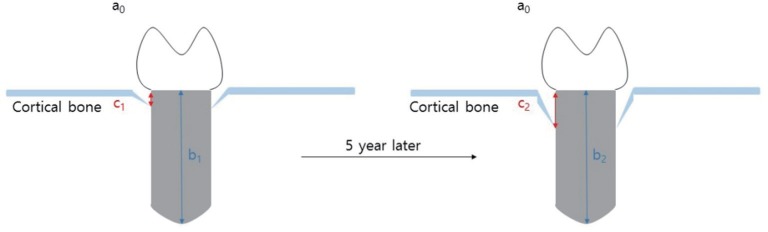 | Fig. 1
Marginal bone loss.
Estimated Level of Marginal Bone = Measured Level of Marginal Bone × Actual Length of FixtureMeasured Length of Fixture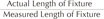 
Marginal Bone Loss = 1st Estimated Value – Final Estimated Value
Actual length of fixture: a, Measured length of fixture: b, Measured level of marginal bone: c,
Estimated level of marginal bone: X=c×ab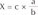
Marginal bone loss = X2 – X1
undefined |
 | Fig. 2Example of measurement using periapical radiographs. (A) Left radiograph was taken after delivering a prosthesis, (B) Right radiograph was taken during follow-up period. Both radiographs are ZM implants in the same patient.
|
Implant stability quotient (ISQ) was measured using the Osstell Mentor (Osstell, Gothenburg, Sweden) device. The initial ISQ was measured immediately after implantation, while the final ISQ was measured during secondary surgery for exposing the implant or during acquisition of prosthetic impression when 1-stage surgery was used for implantation. The mean interval between the two measurements was 135.07 days (OT 145.53 days and ZM 132.65 days). To compensate for the differences in measurement periods, Daily ISQ gain (ISQ/day) index was then obtained and compared between groups.
Successful implantation was determined according to the definition provided by Albrektsson in 1986, which is the criterion that is most often cited:
15 1) no persistent pain, discomfort, or dysesthesia 2) no abscess near the implant 3) no tooth mobility 4) no radio-opacity near the implant 5) show MBL of ≤ 1 mm for 1 year after prosthetic loading 6) show aMBL of ≤ 0.2 mm from 1 year after prosthetic loading 7) show 5-year success rate of ≥ 85% and 10-year success rate of ≥ 80% 8) the percentage of numbers that satisfy these criteria shall be reported as the success rate.
Cumulative survival rate represented the percentage of implants that were not removed from implant placement to the time of final screening.
1617
Data were rearranged using Excel (Microsoft, Redmond, WA, USA) and statistical significance was tested using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Mann-Whitney test was performed on the outcome values after normality test.
Go to :

RESULTS
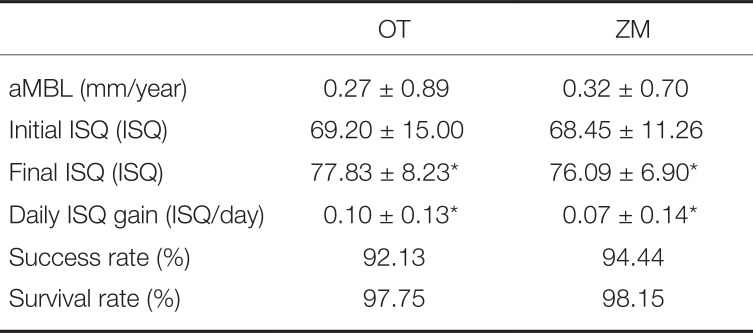
OT and ZM groups did not show statistically significant differences in success rate (
P > .05) and survival rate (
P > .05). With respect to success rate, OT and ZM groups showed 92.1% and 94.4%, respectively. Both groups showed excellent success rates that were higher than the 5-year success rate criterion given by Albrektsson (85%).
15 Meanwhile, aMBLs in OT and ZM groups were 0.27 ± 0.89 mm/year and 0.32 ± 0.70 mm/year, respectively, showing a slightly higher value for OT group, but with no statistically significant difference (
P > .05). Initial ISQ also showed no statistically significant difference between the two groups (
P > .05). The items in which the two implants showed statistically significant difference were final ISQ (
P = .03) and daily ISQ gain (
P = .05). In the final ISQ item, OT and ZM groups showed values of 77.83 ± 8.23 and 76.09 ± 6.90, respectively. OT group showed slightly higher value, and as a result, excellent results were seen in daily ISQ gain (0.10 ± 0.13 > 0.07 ± 0.14), which is an indicator of increase in ISQ rate (
Table 6).
Table 6
Annual bone loss, implant ISQ, success rate, and survival rate (mean ± SD)
|
OT |
ZM |
|
aMBL (mm/year) |
0.27 ± 0.89 |
0.32 ± 0.70 |
|
Initial ISQ (ISQ) |
69.20 ± 15.00 |
68.45 ± 11.26 |
|
Final ISQ (ISQ) |
77.83 ± 8.23*
|
76.09 ± 6.90*
|
|
Daily ISQ gain (ISQ/day) |
0.10 ± 0.13*
|
0.07 ± 0.14*
|
|
Success rate (%) |
92.13 |
94.44 |
|
Survival rate (%) |
97.75 |
98.15 |

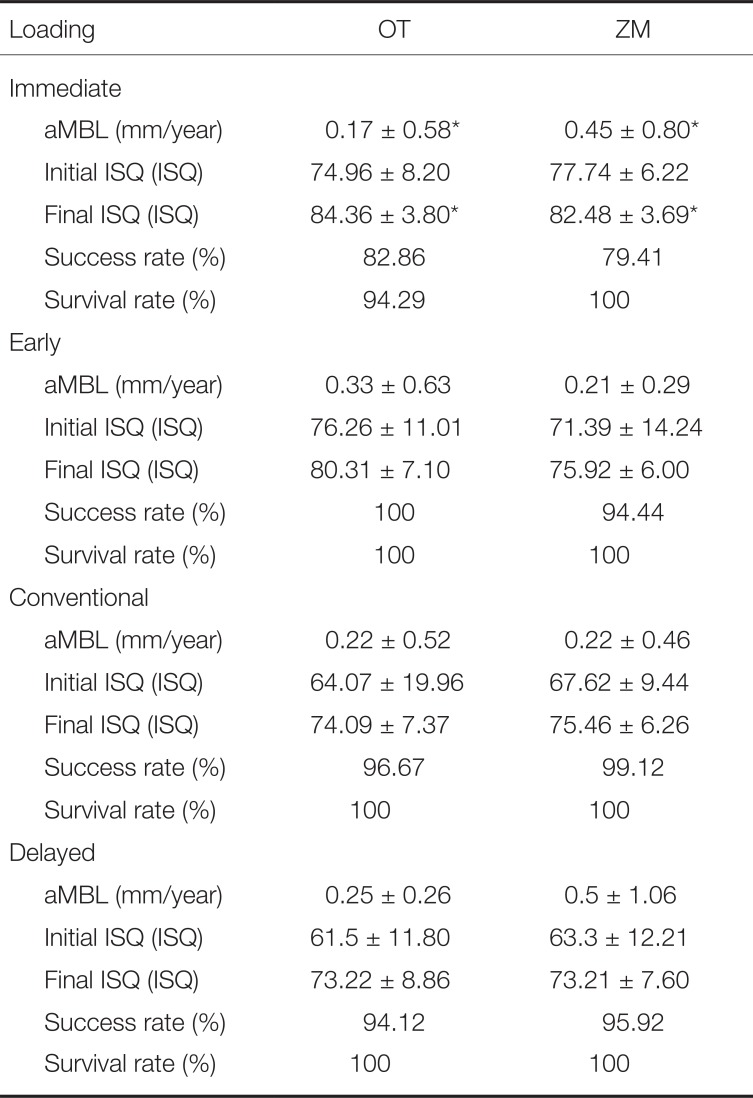
Results based on classification of 4 types of loading indicated that only the group that had immediate loading applied showed statistically significant different results in aMBL (
P = .01) and final ISQ (
P = .01). During immediate loading, aMBL was higher in the OT group (0.17 ± 0.58 mm/year) than in the ZM group (0.45 ± 0.80 mm/year). Similar to the results of the final ISQ index for the same loading method, OT group was marginally higher with 84.36 ± 3.80 ISQ, as compared to ZM group with 82.48 ± 3.69 ISQ. In early, conventional, and delayed loading, OT and ZM groups showed no significant differences (
P > .05;
Table 7).
Table 7
Results specifically classified by loading methods (mean ± SD)
|
Loading |
OT |
ZM |
|
Immediate |
|
|
|
aMBL (mm/year) |
0.17 ± 0.58*
|
0.45 ± 0.80*
|
|
Initial ISQ (ISQ) |
74.96 ± 8.20 |
77.74 ± 6.22 |
|
Final ISQ (ISQ) |
84.36 ± 3.80*
|
82.48 ± 3.69*
|
|
Success rate (%) |
82.86 |
79.41 |
|
Survival rate (%) |
94.29 |
100 |
|
Early |
|
|
|
aMBL (mm/year) |
0.33 ± 0.63 |
0.21 ± 0.29 |
|
Initial ISQ (ISQ) |
76.26 ± 11.01 |
71.39 ± 14.24 |
|
Final ISQ (ISQ) |
80.31 ± 7.10 |
75.92 ± 6.00 |
|
Success rate (%) |
100 |
94.44 |
|
Survival rate (%) |
100 |
100 |
|
Conventional |
|
|
|
aMBL (mm/year) |
0.22 ± 0.52 |
0.22 ± 0.46 |
|
Initial ISQ (ISQ) |
64.07 ± 19.96 |
67.62 ± 9.44 |
|
Final ISQ (ISQ) |
74.09 ± 7.37 |
75.46 ± 6.26 |
|
Success rate (%) |
96.67 |
99.12 |
|
Survival rate (%) |
100 |
100 |
|
Delayed |
|
|
|
aMBL (mm/year) |
0.25 ± 0.26 |
0.5 ± 1.06 |
|
Initial ISQ (ISQ) |
61.5 ± 11.80 |
63.3 ± 12.21 |
|
Final ISQ (ISQ) |
73.22 ± 8.86 |
73.21 ± 7.60 |
|
Success rate (%) |
94.12 |
95.92 |
|
Survival rate (%) |
100 |
100 |

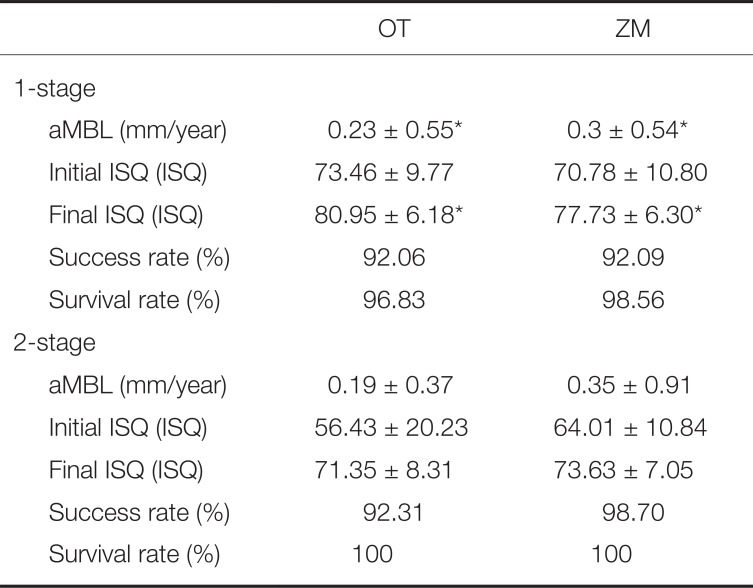
Comparison of results based on dividing the surgical method as 1-stage and 2-stage indicated that OT group showed significantly superior aMBL (
P = .01) and final ISQ (
P = .00) than ZM group for 1-stage surgery. With respect to aMBL index, OT group showed a value of 0.23 ± 0.55 mm/year, which represented less bone resorption by about 0.07 mm/year, as compared to 0.3 ± 0.54 mm/year in the ZM group. With respect to final ISQ index, OT group showed more stable results with 80.95 ± 6.18 ISQ, as compared to 77.73 ± 6.30 ISQ in the ZM group. When 2-stage surgery was applied, OT and ZM groups did not show significant differences (
P > .05;
Table 8).
Table 8
Results specifically classified by surgery method (mean ± SD)
|
OT |
ZM |
|
1-stage |
|
|
|
aMBL (mm/year) |
0.23 ± 0.55*
|
0.3 ± 0.54*
|
|
Initial ISQ (ISQ) |
73.46 ± 9.77 |
70.78 ± 10.80 |
|
Final ISQ (ISQ) |
80.95 ± 6.18*
|
77.73 ± 6.30*
|
|
Success rate (%) |
92.06 |
92.09 |
|
Survival rate (%) |
96.83 |
98.56 |
|
2-stage |
|
|
|
aMBL (mm/year) |
0.19 ± 0.37 |
0.35 ± 0.91 |
|
Initial ISQ (ISQ) |
56.43 ± 20.23 |
64.01 ± 10.84 |
|
Final ISQ (ISQ) |
71.35 ± 8.31 |
73.63 ± 7.05 |
|
Success rate (%) |
92.31 |
98.7 |
|
Survival rate (%) |
100 |
100 |

Initial tooth mobility was the most common early complication. Most cases that showed tooth mobility in the OT group resulted in removal of the implant due to failed osseointegration. Contrarily, osseointegration was successful in the ZM group, except for 2 cases. Other than tooth mobility, biological complications included infection, failed osseointegration, paralysis, and fracture (
Table 9).
Table 9
Early biological complications

|
Early complication type (patients) |
OT |
ZM |
|
Initial tooth mobility |
3 |
7 |
|
Infection |
2 |
3 |
|
Failed osseointegration (removal) |
2 |
2 |
|
Paralysis |
1 |
1 |
|
Fracture |
1 |
0 |
|
Total |
9 |
13 |

Among late stage complications, prosthesis-related complications were most common in both OT and ZM groups, including prosthesis fall out, prosthesis fracture, screw loosening, and implant structure (fixture or abutment) fracture. Prosthesis-related complications were followed in order by peri-implantitis, bone loss, and gingival recession (
Table 10).
Table 10
Delayed complications

|
Delayed complications (patients) |
OT |
ZM |
|
Prosthetic complications |
3 |
3 |
|
Peri-implantitis |
2 |
3 |
|
Bone loss |
3 |
2 |
|
Gingival recession |
0 |
1 |
|
Total |
8 |
9 |

Go to :

DISCUSSION
Achieving rapid osseointegration with long-term stability is a prerequisite for satisfying patients who demand immediate restoration after an implant surgery. For the past 20 years, many researchers have attempted to use hydroxyapatite, a bone component, as coating material to shorten the osseointegration process.
2 Meanwhile, there have been efforts to minimize complications such as peri-implantitis.
567 As a result, recent studies have reported survival rates of 93.2 – 98.5% as clinical outcomes of hydroxyapatite-coated implants.11 Guttenberg reported a 9-year survival rate of 93.3% for Integral (Calcitek Inc., Carlsbad, CA, USA), while Saadoun reported an 8-year survival rate of 96.1% for Steri-Oss (Yorba Linda, CA, USA) implant. Jones reported a 5-year survival rate of 98.5% for Sterngold/implamed (Atteboro, MA, USA) implant.
181920 In the present study, the survival rates in the OT and ZM groups were 97.75% and 98.15%, respectively. In terms of survival rate, these results indicated that the survival rate in the present study represented superior outcome value than studies that used other hydroxyapatite-coated implants. In terms of success rate, OT and ZM groups showed 92.1% and 94.4%, respectively, which represented excellent success rates that were higher than Albrektsson's 5-year success rate of 85%.
15 Meanwhile, the present study clearly distinguished the results on success rates according to Albrektsson's definition of success rate. It holds significance.
A study by Kim in 2013 that used the same implants as the present study examined the outcomes for 12 months, and demonstrated that the clinical outcome of survival rates for both OT and ZM products was 100%, which exceeded 97.75% and 98.15%, respectively, found in the present study. With respect to MBL, the present study showed greater bone resorption than that observed in previous studies, except for the immediate loading OT group.
21 It is believed that this outcome may be attributed to the present study having a longer follow-up period. The mean values were derived with inclusion of results from implants that were not removed despite of severe bone resorption. A 2012 paper by Kim reported success rate of 98.1% and 97.9% for OT and ZM, respectively. This study analyzed 1-year follow-up after implants from two manufacturers were placed by applying immediate loading,
22 which exceeded success rate of 82.86% and 79.41%, respectively, found in the present study. It is believed that this difference was due to difference in the success criteria applied. The 2012 study by Kim reported a case to be successful if MBL showed < 1.50 mm of bone resorption in the first year, whereas the present study determined success as < 1 mm in the first year and < 0.20 mm per year thereafter.
Comparison of results based on loading methods showed that stable prognosis with 100% survival was found in all cases, except in immediate loading cases. This demonstrated that applying loading within 48 hours after implantation can have a critical impact on prognosis. In the OT group, success rate for early loading was 100%, but the rate decreased to 82.86% with immediate loading. In the ZM group, success rate for early loading was 94.44%, which also dropped rapidly to 79.41% with immediate loading. Meanwhile, such rapid change in prognosis was not found between early and conventional loading or between conventional and delayed loading. Consequently, it is still difficult to actively use immediate loading that has uncertain prognosis in actual clinical settings, and thus, it is desirable to apply early loading as an alternative to satisfy the patient's demand for speedy completion of treatment.
In comparison to other studies that examined immediate loading, a study by Polizzi that used the Brånemark implant (Nobel biocare AB, Sweden) reported immediate implant survival rates of 92.4% and 94.7% in the maxilla and mandible, respectively, while OT and ZM coated with HA used in the present study showed a better prognosis with 94.29% and 100%, respectively.
23
Manufacturers of the existing hydroxyapatite use machined surface of about 1 – 2 mm in the upper area of the fixture to prevent plaque buildup or bacterial infection, but OT has a structural difference of being treated with resorbable blasting media (RBM).
24 However, OT and ZM groups in the present study did not show significant differences, and as such, additional studies are needed to determine whether RBM can be used to achieve the same outcome as machined surface.
Comparison between 2-stage and 1-stage surgeries showed that although the surgery itself may be simple, the additional surgery placed burden on the patient and increased the number of hospital visits. The present study compared the prognosis of implant surgeries performed by an experienced surgeon. Survival rates for OT 2-stage and 1-stage were 100% and 96.83%, respectively. Although 2-stage showed a slightly higher survival rate, prognoses were similar for both methods. Survival rates for ZM 2-stage and 1-stage were 100% and 98.70%, respectively, also showing similar prognoses. This demonstrated that when a patient is burdened by having to undergo another surgery, 1-stage surgery may be a stable and better option.
The data are collected from implants which were placed from 2010 to 2012. Some cases were early failure in osseointegration. Some patients stopped during follow-up. The limitation of this study would be the failure to exclude these data. The loading protocol, prosthetic treatment, occlusal restoration, and follow-up period were variable. Additional studies are needed to further investigate long term prognosis of hydroxyapatite-coating implants with refined data.
Go to :

CONCLUSION
In a follow-up study up to 5 years, OT and ZM, hydroxyapatite coated implants, did not show statistically significant differences in success rate, survival rate, and aMBL (P > .05). Success rates for OT and ZM were 92.13% and 94.44%, respectively. OT and ZM showed a high survival rate of 97.8% and 98.2%, respectively, demonstrating that hydroxyapatite-coated implants have very high stability in terms of long-term survival rate. In detail, from the perspective of duration of healing or surgical method, OT showed superior aMBL results than ZM for immediate loading and 1-stage surgery (P < .05). Under other conditions, these two implants did not show any significant difference (P > .05). The significance of the present study was that unlike previous studies that reported clinical outcomes by survival rate only, the present study made comparisons with various indices such as success rate, survival rate, MBL, and ISQ.
Go to :




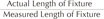
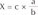







 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download