Abstract
PURPOSE
The marginal bone loss of implants with laser treated surface was investigated after six weeks of loading after implant installation to the mandible molar area.
MATERIALS AND METHODS
A total of 23 implants were placed in the edentulous molar area of the mandible: 13 implants were immediately loaded and 10 implants were early loaded. The implants used were made of titanium grade 23, screw shaped, 4.2 mm in diameter, and 10 mm in length. Patients were evaluated with resonance frequency analysis at implant fixture installation and 1, 2 (final prosthesis installation), 3, 5, 8, and 14 months later. X-rays were taken at 2 months after fixture installation and 1, 2, 3 years after to measure the marginal bone loss.
The main purposes of implant surface treatment are to increase the surface area to obtain a higher mechanical fixation between bone and implant immediately after insertion,1 to provide a surface structure that can maintain a blood clot well,2 and to provide a surface form that can promote the process of bone healing.3 SLActive technique involves forming surface roughness using large grit with the diameter of 250 – 500 µm after sandblasting and etching by hydrochloric acid and sulfuric acid, then washing in a nitrogen state.4
This surface forms a hydroxyl layer and has a high surface energy as a result of contact with water, and increases the ideal contact between the implant and the surrounding factors.4 The activated surface is preserved and stocked in a physiological saline solution to provide to dental clinics.5 The chlorine ions, as anions, and hydroxyl ions are combined to protect the activated surface from air and prevent hydrocarbon binding.678 Based on previous studies, it had been found that these surface properties significantly increased bone to implant contact and resulted in an accelerated healing process of osseointegration during the early stage. This effect leads to enhanced stability of the implant and aids in healing during the critical early stages.91011
According to a recent study, laser treated surface implants help improve the osseointegration process.12 As a unique surface, this method of treating implants prevents contamination with extraneous factors and has a high degree of surface purity, resulting in excellent surface roughness. That is, the entire laser treated surface of the implant has a porous structure that is pure and not contaminated. This porous structure increases the surface roughness and, as a result, enhances the strength of osseointegration.1314
However, there has not yet been a clinical study on the immediate and early loading of implants with laser treated surfaces, which had excellent osseointegration in an animal study.15 Therefore, we applied the technology of laser treatment to an implant surface and conducted clinical trials to investigate if it could be loaded within six weeks after implant insertion.
The study included 15 patients who were recruited and agreed to the clinical trial procedure, and 5 patients were excluded from the study by exclusion criteria. The clinical trial study was completed without any participant drop-out after implant surgery. A total of 23 implants were placed in 15 patients: 13 implants were immediately loaded and 10 implants were early loaded. This study protocol “KHNMC MD IRB 2012-009” was accepted by the Kyung Hee University Hospital at Gangdong, Seoul in South Korea.
All patients received prophylactic antibiotics 2 hours before surgery and their mouths were rinsed with 0.12% chlorhexidine for one minute. A full thickness flap was elevated after local anesthesia. The implants used were made of titanium grade 5, screw shaped, 4.2 mm in diameter, and 10 mm in length (CSM, Daegu, Korea). The implant surfaces were treated with a Nd:YAG laser (Jenoptic Laser Optik, Jena, Germany). Implants were inserted in the edentulous molar area of the mandible, according to Straumann's guidelines on early and immediate loading for SLActive surface implants. In the case of ISQ ≥ 70 and insertion torque value (ITV) ≥ 35 to 50 Ncm at the insertion site, a provisional restoration was connected to the implant within a week after surgery and the final restoration was placed 2 months after surgery for immediate loading. In the case of ISQ ≥ 60 to 70 and ITV ≥ 25 to 35 Ncm at the implant insertion, an impression was taken within 2 weeks after surgery, and the implant received provisional restoration 4 weeks after surgery with a final restoration at 2 months post-op for early loading. After implant placement, each patient received antibiotics for at least three days.
Patients were evaluated with resonance frequency analysis (RFA) at implant fixture installation and also 1, 2 (final prosthesis installation), 3, 5, 8, and 14 months later. Digital bisecting X-rays were taken at 2 months after fixture installation (final prosthesis installation) and then 3, 5, 8, 14, 24, and 36 months later. The digital x-rays were transferred into a software program and the first bone-implant contact (FBIC) was measured on implants for mesial and distal planes. For calibration purposes, the known pitch distance between the implant threads was used. The FBIC was measured for all 23 implants at baseline. The vertical bone loss/gain was calculated as the difference of the bone level (BL) at baseline minus the FBIC at a certain endpoint in each period (3, 5, 8, 14, 24 and 36 months later). An ISQ ≥ 70 and vertical bone loss ≤ 1.5 mm was regarded as a success.
Data are presented as mean ± standard deviation and statistical analysis was performed using repeated-measures ANOVA to identify changes in marginal bone loss over time. All data management and analyses were performed with SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA).
The mean ISQ value measured from surgery was greater than 70 at all-time points (Fig. 1). From implant installation to after 14 months, ISQ values increased gradually. The measured ISQ values at 6 and 12 months after installation in 23 implants were higher than 70, which was the success criterion of this clinical trial.
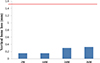
The bone loss values were less than 0.33 ± 0.32 mm after 36 months of implant installation (Fig. 2). These bone loss values are 4.5 times lower than success criteria (≤ 1.5 mm). There was no significant difference between final prosthesis installation and after 12 months. But, except for the two groups, there was a significant difference among all groups.
Studies regarding various surface treatments to enhance osseointegration have led to an increased success rate of implants.2356 The SLA treated surface has excellent biocompatibility and bone affinity.78910 The bone-implant contact of SLA surfaces achieves a high level of osteoblast differentiation occurs actively. It is possible that these properties of the SLA surface influence its osteoconductive ability.11 This virtue could reduce the loading time and increase the possibility of applying early loading.11
Laser treatment of the implant surface rapidly increases the temperature of titanium and melts the structure, consequently increasing the thickness of the oxygen layer.12 After laser treatment, morphological changes and roughness in the titanium surface appear due to the changes in oxygen layer thickness.13 On the surface of laser-treated implant, pre-osteoblast attachment is promoted, pre-osteoblastic differentiation occurs actively, and bioactivity is increased.14 Altered surface roughness helps the adaptation of fibrin and migration of osteoblasts, consequently leading to the deposition of new bone.
In this study, implant prostheses were installed based on insertion torque value; 10 implants were loaded conventionally and 13 implants were loaded immediately. At 6 and 12 months after implant prosthesis installation, 23 implants had an ISQ value greater than 70, which was the success criteria defined in this clinical trial study. Additionally, the average ISQ values, measured five or six times per patient after surgery, were higher than 70 in each of those 23 implants. As another success criterion, the analysis of average value of vertical alveolar bone loss was observed to be less than 1.5 mm, consistent with the goal of this study.
Because the laser treated surface of the implants had significantly superior results compared to SLA surface implants in a previous animal study,13 we performed a clinical trial by early loading, which confirmed the previous results. By measuring the insertion torque when the implants were placed, the immediate loading application to implant was determined. In the immediate loading case, the ISQ values were also higher than 70. This suggests that when higher initial fixation is obtained, results are likely to be successful in either early or immediate loading. Excellent ISQ results over 70 were observed after 6 and 12 months post-insertion, which supports the prediction that a high success rate would be maintained through continuous follow-up. In this study, the average ISQ value for laser treated implants was 81.4 at 24 weeks after implant placement, similar to the results of SLA and SLActive surface of implants. The average bone resorption of 15 patients was 0.33 mm after 36 months, despite different observation periods and research methods from the previous study.
Figures and Tables
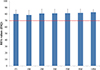 | Fig. 1Mean and SD of ISQ values measured from implant fixture installation to 14 months after surgery. FI: at implant installation, 1M: 1 month later, 2M: 2 months later (final prosthesis placement), 3M: 3 months later, 5M: 5 months later, 8M: 8 months later, 14M: 14 months later. Success criterion was a value greater than or equal to 70 (red-line). |
References
1. Wismeijer D, Casentini P, Gallucci G, Chiapasco M. ITI treatment guide Volume 4, Loading protocol in implant dentistry. Quintessence;2010. p. 6–8.
2. Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006; 76:323–334.

3. Hofmann AA, Bloebaum RD, Bachus KN. Progression of human bone ingrowth into porous-coated implants. Rate of bone ingrowth in humans. Acta Orthop Scand. 1997; 68:161–166.

4. Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005; 74:49–58.

5. Wennerberg A, Galli S, Albrektsson T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin Cosmet Investig Dent. 2011; 3:59–67.

6. Hall J, Miranda-Burgos P, Sennerby L. Stimulation of directed bone growth at oxidized titanium implants by macroscopic grooves: an in vivo study. Clin Implant Dent Relat Res. 2005; 7:S76–S82.

7. Mangano C, Perrotti V, Iezzi G, Scarano A, Mangano F, Piattelli A. Bone response to modified titanium surface implants in nonhuman primates (Papio ursinus) and humans: histological evaluation. J Oral Implantol. 2008; 34:17–24.
8. Schwartz Z, Martin JY, Dean DD, Simpson J, Cochran DL, Boyan BD. Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. J Biomed Mater Res. 1996; 30:145–155.

9. Roccuzzo M, Bunino M, Prioglio F, Bianchi SD. Early loading of sandblasted and acid-etched (SLA) implants: a prospective split-mouth comparative study. Clin Oral Implants Res. 2001; 12:572–578.

10. Cochran DL, Buser D, ten Bruggenkate CM, Weingart D, Taylor TM, Bernard JP, Peters F, Simpson JP. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: early results from clinical trials on ITI SLA implants. Clin Oral Implants Res. 2002; 13:144–153.
11. Cochran D, Oates T, Morton D, Jones A, Buser D, Peters F. Clinical field trial examining an implant with a sand-blasted, acid-etched surface. J Periodontol. 2007; 78:974–982.

12. Hallgren C, Reimers H, Chakarov D, Gold J, Wennerberg A. An in vivo study of bone response to implants topographically modified by laser micromachining. Biomaterials. 2003; 24:701–710.

13. Kang NS, Li LJ, Cho SA. Comparison of removal torques between laser-treated and SLA-treated implant surfaces in rabbit tibiae. J Adv Prosthodont. 2014; 6:302–308.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download