Abstract
STATEMENT OF PROBLEM
The aim of this study was to study the effects of various surface treatments to a titanium surface on the expression of Runx2 in vitro.
MATERIAL AND METHODS
Human Osteosarcoma TE-85 cells were cultured on machined, sandblasted, or anodic oxidized cpTi discs. At various times of incubation, the cells were collected and then processed for the analysis of mRNA expression of Runx2 using reverse transcription-PCR.
RESULTS
The expression pattern of Runx2 mRNA was differed according to the types of surface treatment. When the cells were cultured on the untreated control culture plates, the gene expression of Runx2 was not increased during the experiments. In the case of that the cells were cultured on the machined cpTI discs, the expression level was intermediate at the first day, but increased constitutively to day 5. In cells on sandblasted cpTi discs, the expression level was highest in the first day sample and the level was maintained to 5 days. In cells on anodized cpTi discs, the expression level increased rapidly to 3 days, but decreased slightly in the 5-th day sample.
Many studies have attempted to explain the interaction of bone tissue with various alloplastic biomaterials, such as titanium, that are often used to fabricate dental implants.1,2 Improvement of the integration of biomaterials into bone tissue is one of the challenges in the biomaterials fields. To bring bone tissue integration on implant surfaces various techniques have been used to improve tissue responses to implant surfaces.3-5 Many in vivo and in vitro studies have compared the efficiency of various surface treatments in improving bone tissue integration of implants.4,6 Histological as well as biochemical data from these studies describe a variety of cellular responses to various implant surface conditions.7-12 Many in vitro evaluations of cell responses to implant roughness have been performed in order to discern the surface properties influencing the cell response to implant surface.13-17 To date, comparative studies regarding differences in surface composition and topography effects on cell responses have been scarce.
Runx2 (Cbfa1) is a transcription factor that belongs to the Runx family, and is expressed as two isoforms. Type I and II Runx isoforms are expressed in chondrocytes, as well as osteoblasts, although, type II Runx2 expression is predominant in osteoblasts.18-20 Runx2 binds to an osteoblast-specific cis-acting element, activates the expression of osteocalcin, the most osteoblast specific gene, and regulates osteoblast differentiation and expression of key osteoblast genes necessary for development of a mineralized phenotype. Runx2 plays an essential role in steering multipotent mesenchymal precursor cells toward an osteoblastic lineage21 and promotes osteoblast differentiation at an early stage. However it inhibits osteoblast differentiation at a late stage.22 Runx2 is a positive regulator that can upregulate the expression of bone matrix genes, including type I collagen, osteopontin, bone sialoprotein (BSP), osteocalcin, and fibronectin.20,23,24 Lastly Runx2 was shown to have a role beyond development and differentiation by regulating the rate of bone matrix deposition.23 Thus, Runx2 is a critical gene not only for osteoblast differentiation but also for osteoblast function. However, the effects of different implant surface topographies on gene expression of key osteogenic factors are not fully understood.
The hypothesis of the current study was that different implant surface treatments differentially affect Runx2 gene expression. Readily available Human Osteosarcoma TE-85cells were used. In this study, cells were grown on machined, sandblasted, anodized cpTi discs and control tissue culture plates for 1st, 3rd, and 5th days. The purpose of this study was to address molecular events with respect to the osteogenic key marker, Runx2 gene expression in relation to different implant surface treatments. Using these samples, it is intended to study the different effects of not only surface roughness but also topography on osteoblast gene expression.
TE-85 cells were maintained as sub-confluent monolayers in RPMA 1640 (Gibsco BRL, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) at 37℃. Commercially pure titanium (cpTi) discs with dimensions of 23 mm diameter × 1 mm height were used. The 72 discs' surfaces were prepared and original machined surfaces were used. Among the discs, 24 discs' surfaces were sandblasted with 75 µm Al2O3, while 24 other discs' surfaces were anodized under constant voltage, 350 V. Table I shows the result of the optical interferometer (Acura 2000, Intek Plus, Daejon, Korea) analysis. Sandblasted surface showed rougher surface than anodized one, and anodized surface had rougher surface than machined surface. And surface morphologies were shown in Fig. 1. For each group, 8 titanium discs were placed on a 100 φdish, and TE-85 cells were cultured (1 × 106 cells/mL) on to titanium surfaces with 2 ml 10% FBS growth medium for 1, 3 and 5 days.
Growth media and extra cells (not attached to discs) were suctioned and cells attached to the discs, then washed with PBS solution. Cells were harvested with a hand scrapper. Total cellular RNA was extracted using the RNeasy® Protect kit (Qiagen, Hilden, Germany), DNAse 1-treated then quantified by measuring absorbance at λ260 nm on a UV160U spectrophotometer (RB-10. Dynamica, Salzburg, Austria). For the first strand cDNA synthesis, an initial RT mixture was treated [2 µg total RNA, 1 mM dNTPs, 50 pmol Poly (dT)-15, 30 mM KCl, 8 mM MgCl2, and 1 mM dithiothreitol, in 25 mM Tris-HCl] and incubated at 65℃ for 5 minutes, then quenched on ice.
10µl of diluted cDNA was transferred into a 10 µl PCR reaction mixture that contained 5 pmol/µl of sense and antisense oligonucleotide primers, 1×PCR buffer (10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2), 0.1 mM dNTPs, and 0.5 U TaqDNA polymerase. The forward primer (5-TCTGGCCTTCCACTCTCAGT-3) and reverse primer (5-TATGGAGTGCTGCTGGTCTG-3) of Runx2 were synthesized based on the Runx2 mRNA sequence. Amplification reactions for the Runx-2 cDNA and the house keeping gene glyceraldehydes phosphate dehydrogenase (GAPDH) were carried out. Amplifications were performed using an Authorized thermal cycler (eppendorf, Mastercycler gradient, Hamburg, Germany), with the temperature cycling being set as follows: 94℃ for 60 s, 58℃ for 30 s, 72℃ for 60 s: 5 cycles, 94℃ for 30 s 56℃ for 30 s, 72℃ for 60 s: 5 cycles, 94℃ for 30 s, 54℃ for 30 s, 72℃ for 60 s: 25 cycles, followed by a final extension at 72℃ for 10 minutes.
PCR products were analyzed by 1% agarose gel electrophoresis containing 0.01% ethidium bromide. Visualized PCR product bands were sliced from the gel and fluorescence within the gel was detected using a Lumi-imager F1 Workstation (Roche Molecular Biochemicals, Indianapolis, IN, USA). The relative intensity of each band was determined and plotted as the relative abundance of Cbfa1/GAPDH amplification product abundance.
The expression of Runx2 gene was examined using Reverse transcription PCR and the electrophoresis result is shown in Fig. 2. Runx2 expression differences in TE-85 cells over a 5-th day time course with varied implant surface treatments are shown in Fig. 3. The results demonstrate more Runx2 expression in cells grown on sandblasted surface at the first day of culture, on anodic oxidized surface at 3-rd day of culture, on machined surface at 5-th day of culture. The patterns of the gene expression with different surface treatment were noted along the time table. The Runx2 mRNA from cells cultured on the control plate increased to 3 days and at a constant level to 5 days of culture. mRNA from cells cultured on machined surface discs increased consistently to 5 days. mRNA from cells cultured on sandblasted surface discs showed highest level expression on the first day, then remained constant during the 5-th day of culture period. mRNA from cells cultured on anodized surface discs increased rapidly from the first day to the third day and then slightly decreased at the fifth day of culture (Table II).
Osteoblast differentiation, ECM formation, and subsequent mineralization are needed for bone formation associated with osteogenesis and subsequent osseointegration. Transcription factor Runx2 regulates this development. Osteoblast differentiation and responses during osseointegration vary and are affected by the implant surface microtopography, associated extracellular matrix proteins, and their respective integrin receptors.25-29 A lot of studies in examining cell adhesion and morphology, DNA synthesis, integrin and extracellualar matrix expression, and enzyme activity have been done to elucidate osteoblastic response to titanium alloys.26,30-32 However, many of the molecular and genotypic events taking place at the osteoblast cell level during osseointegration are still largely unknown.
The purpose of our study was to address these molecular events with respect to the osteogenic key marker, and Runx2 gene expression in relation to different implant surface treatments. In this study, the amounts and patterns of Runx2 gene expressions were different with time according to various surface treatments. Various hypotheses could be proposed to explain these data.
Roughness increased from tissue culture plate, machined discs, anodized discs and sandblasted discs respectively. During the study period, levels of Cbfa1 expression increased with increasing roughness. From this result, we could hypothesize that the rougher the implant surface, the sooner the Runx2 gene expresses. The early expression of Runx2 in TE-85 cells on cpTi discs of increased surface roughness or topographic complexity is congruent with in vivo observations regarding the extent of osteogenesis on implants of increasing surface roughness.9 The surface properties of an implant seem to influence the components of the cell cytoskeleton involved in cell spreading and locomotion. Another determinant of cell shape and spreading onto a surface is the establishment of cell contacts and adhesion to the surface. Cell contact and adhesion are time-dependent phenomena and many studies support early spreading of osteoblasts on rougher surfaces.7,10 Aside from the direct effects of the cytoskeleton, integrin-mediated signaling pathways are known to affect gene expression, as well as increased Runx2 gene expression was noted on rougher surfaces.15 Another study reported that differentiation of preosteoblasts is affected by implant surface topographies.16 Similarly our data indicated that early Runx2 gene expression was favored by the rougher surface.
Second, the expression amount of the gene should be considered. In this study the highest expression was recorded on the machined surface at the fifth day of culture. However we should consider not only the amount, but also the timing of expression and the activity of Runx2. Shui et al. demonstrated that human bone marrow-derived mesenchymal stem cells constitutively express Runx2. However there was a lack of correlation between Runx2 mRNA or protein levels and the acquisition of the osteoblastic phenotype in these cells.18 Franceschi et al. demonstrated that Runx2 overexpression in immature osteoblast-like cells resulted in the acceleration and robust up-regulation of matrix mineralization.33 In this study osteosarcoma TE-85cells were used, which are committed osteoblasts that are more mature osteoblast compared to preosteoblast or bone marrow stromal cell. In this cell line, early expression and activity of Runx2 during that time could be more meaningful. The amount of expression itself could be critical for accelerating and enhancing osseointegration. However, additional studies about the activity of Runx2 related to surface treatments in these cells are strongly recommended.
Third, the effect of not only roughness but also chemical composition and the characteristics of surface microstructure on Runx2 expression should be considered. Previous studies supported and established the effects of anodic oxidized treatment showed improvement of osseointegration and biologic responses. The microstructure along with phase and composition of oxide layers are significantly changed by micro arc oxidation (MAO). The concentrations of Ca or P ions in the oxide layer are increased with the applied voltage.6 Furthermore, the changes in chemical composition and roughness of the Ti surface played crucial roles in the biocompatibility of the implant. Li et al. demonstrated roughness and the amount of Ca and P ions incorporated into the titanium oxide layer strongly affect the cell response.6 This study showed that different implant surface microtopographies (machined, sandblasted, anodic oxidized) may alter the expression of key osteogenic regulatory genes such as Runx2. This suggests that the interaction of osteoblasts with the extracellular matrix components on different implant surface microtopographies can influence gene expression. Perhaps this occurs as a result of differences in cell adhesion and shape, as a result of integrin-mediated adhesion and regulation of downstream signaling cascades as reported.26 It could also be a result of extracellular matrix spatial and temporal expression profile changes that would appear under the control of the transcription factor Runx2, such as bone sialoprotein (BSP2).20
Thus, different surface treatments may contribute to the regulation of osteoblast differentiation by influencing the level of gene expression of key osteogenic factors. A better understanding of these molecular processes will lead to the development of more advanced therapeutic interventions associated with dental implant therapy and tissue-engineering biological applications. In future, we could use not only mechanical or chemical treatment to improve osseointegration, but also gene therapy-based strategies for bone regeneration may be achieved using the approach of over-expressing combinations of factors for improving the extent and type of bone formed in regenerating sites.
Human osteosarcoma TE-85 cells were cultured on machined, sandblasted and anodic oxidized cpTi discs. After the first, the third, and the fifth days cells were harvested and reverse transcription PCR was used for comparative analysis of Runx2 gene expression to study the effect of various surface treatments of titanium surface on the expression of Runx2 in vitro. The results were as follows.
1. More expression of Runx2 in cells grown on sandblasted surface at the one-day culture were observed; on anodic oxidized surface at the three-days culture, and on machined surface at five-days culture was noted.
2. In cells on tissue culture plates, the lowest expression level was noted, and the level increased slightly by 3 days, and was maintained to 5 days.
3. In cells on machined cpTi discs, the expression level was intermediate at the first day, however increased constitutively to the fifth day.
4. In cells on sandblasted cpTi discs, the expression level was highest in the 1 day sample and the level was maintained to 5 days.
5. In cells on anodized cpTi discs, the expression level increased rapidly to 3 days, but decreased slightly in the 5 day sample.
We can conclude that different surface treatments may contribute to the regulation of osteoblast differentiation by influencing the level of gene expression. However, it is considered that future studies with more controlled conditions and experimental samples are necessary to understand the mechanism of cellular responses to different implant surface treatments.
Figures and Tables
 | Fig. 1SEM (JSM-840A, JEOL, Japan) of the machined, sandblasted and anodized surface structures of the prepared titanium discs. |
 | Fig. 2Effects of surface treatments on Runx2 mRNA expression in TE-85 cells. Ehidium bromide-stained agarose gel analysis of RT-PCR products. p: control tissue culture plate. m: machined. a: anodic oxidized. s: sandblasted. |
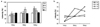 | Fig. 3a, b. Reverse transcription PCR analysis for Runx2 gene expression by TE-85 cells over time (in days). The amounts and patterns of Runx2 expressions differ in time according to various surface treatments (p: tissue culture plate, m: machined, a: anodic oxidized, s: sandblasted). Values represent Runx2/GAPDH intensity. |
References
1. Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003. 67:932–949.
2. Schwartz Z, Kieswetter K, Dean DD, Boyan BD. Underlying mechanisms at the bone-surface interface during regeneration. J Periodontal Res. 1997. 32:166–171.
3. Wennerberg A. Implant design and surface factors. Int J prosthodont. 2003. 16:45–51.
4. Bigerelle M, Anselme K, Noel B, Ruderman I, Hardouin P, Iost A. Improvement in the morphology of Ti-based surfaces: a new process to increase in vitro human osteoblast response. Biomaterials. 2002. 23:1563–1577.
5. Ratner BD. Replacing and renewing: synthetic materials, biomimetics, and tissue engineering in implant dentistry. J Dent Educ. 2001. 65:1340–1349.
6. Li LH, Kong YM, Kim YW, Kim HE, Heo SJ, Koak JY. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004. 25:2867–2875.
7. Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulation bone and cartilage cell response. Biomaterials. 1996. 17:137–146.
8. Xavier SP, Carvalho PS, Beloti MM, Rosa AL. Response of rat bone marrow cells to commercially pure titanium submitted to different surface treatments. J Dent. 2003. 31:173–180.
9. Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992. 7:302–310.
10. Ong JL, Prince CW, Raikar GN, Lucas LC. Effect of surface topography of titanium on surface chemistry and cellular response. Implant Dent. 1996. 5:83–88.
11. Schneider G, Burridge K. Formation of focal adhesions by osteoblasts adhering to different substrata. Exp Cell Res. 1994. 214:264–269.
12. Masuda T, Salvi GE, Offenbacher S, Felton DA, Cooper LE. Cell and matrix reactions at titanium implants in surgically prepared rat tibiae. Int J Oral Maxillofac Implants. 1997. 12:472–485.
13. Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999. 13:38–48.
14. Carinci F, Pezzetti F, Volinia S, Francioso F, Arcelli D, Marchesini J, Scapoli L, Piattelli A. Analysis of osteoblast-like MG63 cells' response to a rough implant surface by means of DNA microarray. J Oral Implantol. 2003. 29:215–220.
15. Schneider GBH, Perinpanayagam H, Clegg M, Zaharias R, Seabold D, Keller J, Stanford C. Implant surface roughness affects osteoblast gene expression. J Dent Res. 2003. 82:372–377.
16. Schneider GB, Zaharias R, Seabold D, Keller J, Stanford C. Differentiation of preosteoblasts is affected by implant surface microtopographies. J Biomed Mater Res A. 2004. 69:462–468.
17. Ogawa T, Sukotjo C, Nishimura I. Modulated bone matrix-related gene expression is associated with differences in interfacial strength of different implant surface roughness. J Prosthodont. 2002. 11:241–247.
18. Shui C, Spelsberg TC, Riggs BL, Khosla S. Changes in Runx2/Cbfa1 expression and activity during osteoblastic differentiation of human bone marrow stromal cells. J Bone Miner Res. 2003. 18:213–221.
19. Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RJ. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the osf2 transcription factor. J Biol Chem. 1998. 273:32988–32994.
20. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997. 89:747–754.
21. Komori T. A fundamental transcription factor for bone and cartilage. Biochem Biophys Res Commun. 2000. 276:813–816.
22. Komori T. Runx2, A multifunctional transcription factor in skeletal development. J Cell biochem. 2002. 87:1–8.
23. Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999. 13:1025–1036.
24. Prince M, Banerjee C, Javed A, Green J, Lian JB, Stein GS, Bodine PV, Komm BS. Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. J Cell Biochem. 2001. 80:424–440.
25. Ducy P. Cbfa1: a molecular switch in osteoblast biology. Dev Dyn. 2000. 219:461–471.
26. Schneider GB, Zaharias R, Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res. 2001. 80:1540–1544.
27. Byers BA, Pavlath GK, Murphy TJ, Karsenty G, Garcia AJ. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfal. J Bone Miner Res. 2002. 17:1931–1944.
28. Brett PM, Harle J, Salih V, Mihoc R, Olsen I, Jones FH, Tonetti M. Roughness response genes in osteoblasts. Bone. 2004. 35:124–133.
29. Ziros PG, Gil AP, Georgakopoulos T, Habeos I, Kletsas D, Basdra EK, Papavassiliou AG. The bone-specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J Biol Chem. 2002. 277:23934–23941.
30. Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. Int J Oral Maxillofac Implants. 2000. 15:675–690.
31. Sinha RK, Tuan RS. Regulation of human osteoblast integrin expression by orthopedic implant materials. Bone. 1996. 18:451–457.
32. Puleo DA, Holleran LA, Doremus RH, Bizios R. Osteoblast responses to orthopedic implant materials in vitro. J Biomed Mater Res. 1991. 25:711–723.
33. Franceschi RT. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med. 1999. 10:40–57.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download