Abstract
STATEMENT OF PROBLEM
A few of studies which compared and continuously measured the stability of various surface treated implants in the same individual had been performed.
PURPOSE
We aim to find the clinical significance of surface treatments by observing the differences in the stabilization stages of implant stability.
MATERIAL AND METHODS
Eight different surface topographies of dental implants were especially designed for the present study. Machined surface implants were used as a control group. 4 nano-treated surface implants (20 nm TiO2 coating surface, heat-treated 80 nm TiO2 coating surface, CaP coating surface, heat treated CaP coating surface) and 3 micro-treated surface implants [resorbable blast media (RBM) surface, sandblast and acid-etched (SAE) surface, anodized RBM surface] were used as experiment groups. All 24 implants were placed in 3 adult dogs. Periotest® & ISQ values measured for 8 weeks and all animals were sacrificed at 8 weeks after surgery. Then the histological analyses were done.
RESULTS
In PTV, all implants were stabilized except 1 failed implants. In ISQ values, The lowest stability was observed at different times for each individual. The ISQ values were showed increased tendency after 5 weeks in every groups. After 4 to 5 weeks, the values were stabilized. There was no statistical correlation between the ISQ values and PTV. In the histological findings, the bone formation was observed to be adequate in general and no differences among the 8 surface treated implants.
Reducing the treatment period in recent implant dentistry is a matter of main concerns. There are so many factors affecting the success rate of immediate or early loaded implants. Factors such as material biocompatibility, implant designs and surfaces, surgical technique, host bed, and the loading conditions have all been shown to influence implant osseointegration.1-4
In these days, numerous studies about surface treated implants have been conducting.7 The implant surfaces and types most frequently described in the literature and used in medical practice may be subdivided into implants with roughened surfaces by coating [e.g. resorbable blast media (RBM), hydroxyappatite coated (HA)], implants with the roughened surfaces with electrochemical modifications (anodic oxidation) of the commercially pure titanium, and implants with the roughened surfaces without coating [e.g. sand-blasted or acid-etched]. The macroscopic and microscopic features of implant surfaces have been described as major factors of osseointegration. Consequently, modifications in the implant body design and implant surfaces have been suggested to increase the success in the poor quality bone by, hypothetically, gaining better anchorage and providing more surface area of load to decrease stress to the softer bone types.15-20
To impart bioactivity to Ti and enhance bone growth, surface treatments such as surface roughening by sand blasting, formation of anatase phase TiO221, hydroxyapatite (HAp) coating, or chemical treatment22-25 have been utilized. Webster et al.26-27 reported that a creation of nanostructure on a ceramic material such as aluminum oxide with grain/particle size of less than - 100 nm regime significantly improved bioactivity of implant and enhanced osteoblast adhesion. Oh et al. suggested,28 TiO2 nanotube arrays and associated nanostructures could be useful as the well-adhered bioactive surface layers on Ti implant metals and alloys for orthopedic and dental applications. Karlsson et al.29 suggested, the anodized nano-porous alumina membranes seem to provide better surface for osteoblastic cell growth, with cells rapidly spreading, flattening and adhering firmly to the surfaces of the materials.
In the past, objective measurements of the stability have been proposed with several methods like the Periotest®. However, they have been criticized because of their lack of resolution, poor sensitivity and susceptibility to operator variables. Recently, resonance-frequency analysis has been introduced to achieve an objective measurement of implant primary stability and to monitor implant stability in the long term.6-12 With resonance frequency analysis, it is possible to measure the degree of implant stability at any time during the course of implant treatment and loading.
We aim to find the clinical significance of surface treatments by comparing stabilization stages of implants in dogs. The ISQ and Periotest® were measured each week in the period of 8 ones from 8 various kinds of implants having different implant surfaces placed in the mandibles of 3 adult dogs.
Eight different types of dental implants were designed for the present study (Table I and Fig. 1). Implants in machined surface group produced by a Company (ExFeel®, MEGAGEN, Korea). Implants surfaces in nano-treated surface groups were treated by sputtering method in laboratory. For group 2) and 4), the sputtering parameters are as follows: 300 W, 1.0 - 1.2 × 10-2 torr, for 3 hours. Group 4 is heat-treated at 600℃ for 1 hour after deposition. For group 3) and 5), the sputtering parameters are as follows: dental implants were coated by sputtercoated using a CMS-18 radiofrequency magnetron sputtering system (Kurt J. Lesker Company, Clairton, PA, USA). The machine was operated at 300 W, 1.0 - 1.2 × 10-2 torr, for 7 hours. Pins were rotated 120 degree between each of three coated periods to cover the entire 360° surface of the specimens. CaP pins were subjected to a post coated heat treatment of 600℃ for 1 hour to achieve 60% crystallinity. Implants surfaces in micro-treated surface groups were produced by a company (ExFeel®, MEGAGEN, Korea). For group 6, 7 and 8, sanding conditions are as follows: MCD apatitic abrasive (Hi-Med Co, USA) was used under bout 150 mesh, 5 atm condition. For group 7, the etching conditions are as follows: in etching solution (HCl : H2SO4 = 4 : 1) group 7 is treated at 80℃ for 5 minutes after blasting. For group 8, anodizing conditions are as follows: in an electrolytic solution dental implants were dissolved calcium and phosphate in water. The electrolytic voltage was set in the range of 170 - 320 V and the current density was 30 mA/cm2. Total 24 implants, 3.75 mm diameter × 10 mm length, were installed by self-tapping.
Three healthy adult dogs, with body weight ranging from 15 to 20 kg were used in this study. The dogs were anesthetized with the combination of ketamine (5 ml/kg, Yu-han, Gunpo, Korea) and Rompun (0.3 ml/kg, Bayer Korea, Ansan, Korea) intramuscularly.
Mandibular premolars were extracted bilaterally. 5 months later, both sides of mandible were prepared in the standard sterile fashions. An incision was made on the top of the alveolar crest extending from the canine to the 1st molar. Gingiva was dissected to allow the elevation of the muco-periosteal flaps. The preparation sites were marked using a round drill, and the fixture sites were prepared. All implants were 3.75 mm in diameter and were larger than the final drill sizes, that was 3.3 mm in diameter. Eight implants were placed in order at each mandible bilaterally (Fig. 2).
The surgical site was closed with resorbable suture materials (SURGIFIT, AILEE company limited, Seoul, Korea). All animals received 0.155 ml/kg Baytril® 50 injection (Bielkorea, Korea) for 7 days and 0.15 ml/kg Pirin® (Green Cross Veterinary Products Co, Korea) for 2 days intramuscularly.
All animals were sacrificed at 8 weeks after surgery with the overdose of thiopental sodium (sigma, USA).
The experiment schedule for the measurement of implant stability is shown in Fig. 3.
After the ISQ values measured, Periotest® measurements (Siemens, Bensheim, Germany) were performed. The handpiece was held at a distance of 2 mm from the healing abutment surface. The percussion of the implant with the rod was performed perpendicularly to the longitudinal axis of the implants and orthoradically to the arch at the coronal platform of the buccal healing abutment surfaces. For each implant, 2 measurements of PTV (Periotest Value) were performed.
ISQ (implant stability quotient) is recorded as a number between 1 and 100, 100 is representing the highest degree of stability. Each transducer is calibrated by the manufacturer, which makes all measurements directly comparable. In order to perform the resonance frequency analysis measurements, the placed healing abutments were removed (Osstell™, Integration Diagnostics, Gothenburg, Sweden). The transducer was mounted on the implants orthoradially with the upright part on the oral side. It was tightened with a screw by hand.
The implants and surrounding bones were fixed in the neutral buffered formalin, dehydrated with ascending concentrations of ethanol for 24 hours at the each stage. Following transitional acetone immersion, the samples were immersed in 100% polymethylmethacrylate monomer for 24 hours, followed by immersion in a 1 : 1 ratio of polymethylmethacrylate to methylmethacrylate monomer for 24 hours. The samples were placed in embedding molds containing polymethalmethacrylate resin for 24 hours. Thereafter, the samples were transferred to fresh methylmethacrylate and bench top-cured at room temperature for 10 days. Once the plastic was hardened, the samples were placed into a 37℃ oven for final curing for 7 to 10 days. The samples were serials sectioned with a Buehler Isomet saw (Buehler, Lake Bluff, IL) using diamond wafering blades at the initial thicknesses of 150 µm. The sections were hand-ground with diamond disks to a final thickness of approximately 30 to 50 um for subsequent analysis. In this manner, 3 to 4 sections were obtained buccolingually for the implants. The sections were stained with hematoxylin and eosin.
Photomicrographs for histological analyses were taken using a Leitx Orthoplan microscope at various magnifications (Eclipse 80i, Nikon, Japan). For 8 weeks, the overall bone formation was observed. From the failed implants, we analyzed the histological difference.
The statistical analysis of the differences of PTV and ISQ values were analyzed by two-way ANOVA and for post hoc comparison Duncan's test was performed. The correlationship between the differences in the ISQ values and PTV were analyzed by linear regression analyses. P-values equal to or smaller than 0.05 were considered to be significant. All calculations were made with SPSS Version 12 for Windows.
The values differences for the individual characteristics were large, stability was compared in each case.
The PTV increased till 4 weeks and then had the decreasing tendency. The ISQ values were minimal after 1 post operative week and then they had the increasing pattern. It had the increasing tendency after 4 weeks and then showed the stable tendency at 5 weeks after operation (Table II and III, Fig. 4).
Fig. 7 illustrates a ground-section of an implant with surrounding hard tissues. The peripheral portions or the pitches are in close contact with the surrounding tissue.
The bone tissue formation is located at both the cervical region of implants and the underneath region. The newly-formed bone is matured and connected well with the around mandible.
The PTV had the negative values except failed implants, but there was no statistical meaning of the correlationship between the differences in the ISQ values and PTV.
Although a good interexaminer reliability was reported, a number of variables influenced the PTV. They could be increased or decreased by changes in the vertical measuring point on the implant abutment, the handpiece angulation and the horizontal distance of the handpiece from the implant. Therefore, the use of the resonance frequency analysis device seems to be safer in assessing reliable implant stability data, because variables during the standardized measurements are kept to a minimum.8
The ISQ values have been claimed to be useful for monitoring implant osseointegration during the healing phase.8 However, few studies describing normal ISQ values have been published. The implicit assumption is that implants undergoing osseointegration are supposed to increase their stability with time or at least maintain it (Meredith 1998).9-11 An ISQ level of 69 (range of 57 - 82) may describe the stability of a fully integrated implant.12-15 The RFA method, as a diagnostic tool, was not reliable in identifying mobile implants, however implant stability could be reliably determined for implants with an ISQ ≥ 47.16
In this study, no significant difference was found from implant stability (PTV, ISQ values) between various implant surfaces. But, as shown in Fig. 7 - 9, the measured stability increased together with the healing process in all implants. The measured ISQ values did not change significantly or increase with time after 5 weeks. This means that the implants had reached a stable state. Clinically, the stability of a dental implant system increases as the bone contact increases. After the osseointegration process has stabilized, stability of the implant system is achieved; therefore the ISQ values reach a plateau.
Furthermore the lowest ISQ values were seen between 2 to 4 weeks after implant placement. Other study indicated that the initial resorption periods at implant surgery were found in the first 2 weeks.7 In an in vivo study of de novo alveolar bone formation adjacent to endosseous implants, described a novel model to investigate different temporal phases of wound healing that result in osseointegration.17 In this animal model, new bone formation was noted at 1 week postplacement. Replacement of the original bone that was responsible for the initial stability of the implant at placement was well underway at the 2 week mark.
A rough estimate of comparative healing rates between dogs and humans would suggest that the events of wound healing and bone remodeling happen approximately 1.5 times sooner in dogs than would occur in the human. In this study during the process of healing, the ISQ values of the tested implants increased and reached a plateau at around 5 weeks. Although no human data are available from this study, the ISQ curve for humans can be predicted to reach a plateau at around 7 weeks or 8 weeks.
When the implants failed the ISQ values decreased. This is because the boundary condition of the implants was destroyed and, therefore, the contact area at the implant-bone interface was reduced.
In this study, we did final drilling till 3.3 mm to increase the initial fixation forces, so that the ISQ values after 8 weeks were higher than initial ISQ values. An implant with a better initial stability condition (i.e. with higher initial ISQ values) would result in a higher final ISQ values.4-5 Higher initial ISQ values had shorter simulated healing times.6 But, in one individual, the stability decreased in gap after one week was big because the initial fixation force was so great that there was the cortical bone ischemia. And the stability after 8 weeks was lower than initial stability.
In the histological findings there was no specific infection that meant the good osseointegration. In a study in vivo, at 8 and 12 weeks, there were marked signs of remodeling within the wound chamber.17 Consequently in the histological view, it means that the implants of this study has no mechanical, functional problem on the loading after 8 weeks. But the histological view should be analyzed earlier because the ISQ values of the implants loading time meant the 5 or 6 weeks.
The process of osseointegration is affected by many factors, including surgical techniques and the conditions of the implant bed.3 Clinical observations have also indicated that the final healing time is affected by individual differences and operation conditions.18 The results given here also demonstrated that the initial ISQ values of implants vary with each individual. It could be concluded that from all difference tendency of this study, the variation of surgical situation (e.g. bone quality, bone quantity, insertion torque, heat generation etc) may be one of the major factors to estimate the clinical success. Therefore, the test implants were evaluated individually.
One of the implants was lost during the experimental periods. The x-ray view showed that the implants invaded the nerves in this study. It was assumed that produced paralysis then maintained for 8 weeks, and made overloaded the occlusal force. So it could be suggested that the reason of the visible failure of implants which produced clinical instability.
Also in this study, the implants were planted always same place with the same sequence so that could effect the results. The purpose of this study is not the comparison of the values according to the implant surfaces but the timing of the osseointegration. We wanted to find the changes of the stability of each implant so the problem of the places of implants was not considered.
It is well known that rapid ingrowth of bone into the threads during the initial healing period make sure better stability of the implants after the 1st surgery and also contributes to advance the long-term success.19-23 However, further study for the proper healing time and mechanics by the surface properties of implant is needed.
1. In PTV, all implants were stabilized except 1 failed implant. (The PTV was in negative values)
2. In ISQ values,
1) The lowest stability was observed at different times for each implant.
2) The ISQ values showed increased tendency after 5 weeks in every groups. After 4 to 5 weeks, the values were stabilized.
3) In one animal (dog 1), the decreased gap of stability was big after 1 week. But at 8 weeks the stability was increased so that most of the groups had over 68 ISQ values except 1 implant (heat-treated 80nm TiO2 coating surface).
4) In another 2 animals, the ISQ values after 8 weeks were higher than the initial ISQ values.
3. There was no statistical correlation between the ISQ values and PTV.
4. In the histological findings, the bone formation was observed to be adequate in general and no differences among the 8 surface treated implants.
In the limitation of this study, the difference in the stability of the implants was determined not by the differences in the surface treatment but by the individual specificity.
Figures and Tables
 | Fig. 1Eight fixtures with different treated implant surfaces. (starting from the left) machined surface (a), 20 nm TiO2 coating surface (b), CaP coating surface (c), 80 nm TiO2 coating surface (d), heat treated CaP coating surface (e), resorbable blast media (RBM) surface (f), sandblast & acid-etched (SAE) surface (g), anodized RBM surface (h). |
 | Fig. 2(a) Implants were placed in order and healing abutments were connected. (b) Implant placement diagram. Placement of 8 implants at each mandible took place from the left posterior side in the same fashion. |
 | Fig. 3The experiment schedule. 5 months before implant placement, the tooth was extracted. Periotest & ISQ values were measured each week in the period of 8 weeks. |
 | Fig. 4PTV (periotest value, PTVs) & ISQ (implant stability quotient, Hz) values in diagram (dog 1). (a) Generally the PTV were increased at the starting and then decreased. In addition, the ISQ values showed the opposite patterns. (b) They gave the minimal values at 1st week meaning that each value decreased in the large scales. Having high initial values also showed high in numbers after 8 weeks. |
 | Fig. 5PTV & ISQ values in diagram (dog 2). (a) Generally the PTV were increased at the starting and then decreased. (b) The ISQ values showed the increasing tendency after 4 weeks and then showed the stable tendency at 5 weeks.
Refer to Fig. 4. for abbreviations
|
 | Fig. 6PTV & ISQ values in diagram (dog 3). (a) Generally the PTV were increased at the starting and then decreased. (b) The ISQ values showed the increasing tendency after 4 weeks. Refer to Fig. 4. for abbreviations |
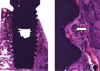 | Fig. 7Histological sections in the buccolingual direction in light microscopic (LM) pictures, showing newly-formed bone was matured and connected well with the around mandible. |
 | Fig. 10A ISQ values diagram in the failed implants. The ISQ values had the decreasing tendency after 5 weeks. |
Table III
ISQ (implant stability quotient) values in the dog 1 (Hz)

Refer to Table II for abbreviations
References
1. Albrektsson T, Lekholm U. Osseointegration: current state of the art. Dent Clin North Am. 1989. 33:537–554.
2. Ericsson I, Johansson CB, Bystedt H, Norton MR. A histomorphometric evaluation of bone-to-implant contact on machine-prepared and roughened titanium dental implants. A pilot study in the dog. Clin Oral Implants Res. 1994. 5:202–206.
3. Schatzker J, Horne JG, Sumner-Smith G. The effect of movement on the holding power of screws in bone. Clin Orthop Relat Res. 1975. 111:257–262.
4. Huang HM, Chiu CL, Yeh CY, Lin CT, Lin LH, Lee SY. Early detection of implant healing process using resonance frequency analysis. Clin Oral Implants Res. 2003. 14:437–443.
5. Parel SM, Triplett RG. Immediate fixture placement: a treatment planning alternative. Int J Oral Maxillofac Implants. 1990. 5:337–345.
6. Meredith N, Friberg B, Sennerby L, Aparicio C. Relationship between contact time measurements and PTV values when using the Periotest to measure implant stability. Int J Prosthodont. 1998. 11:269–275.
7. Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998. 11:491–501.
8. Meredith N, Book K, Friberg B, Jemt T, Sennerby L. Resonance frequency measurements of implant stability in vivo. A cross-sectional and longitudinal study of resonance frequency measurements on implants in the edentulous and partially dentate maxilla. Clin Oral Implants Res. 1997. 8:226–233.
9. Meredith N, Shagaldi F, Alleyne D, Sennerby L, Cawley P. The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clin Oral Implants Res. 1997. 8:234–243.
10. Sennerby L, Roos J. Surgical determinants of clinical success of osseointegrated oral implants: a review of the literature. Int J Prosthodont. 1998. 11:408–420.
11. Sennerby L, Meredith N. Resonance frequency analysis: measuring implant stability and osseointegration. Compend Contin Educ Dent. 1998. 19:493–498. 500502quiz 504.
12. Park CJ. A study on the change of implant stability using resonance frequency analysis. SNUDH Dept of Pros. Unpublished 2003.
13. Nedir R, Bischof M, Szmukler-Moncler S, Bernard JP, Samson J. Predicting osseointegration by means of implant primary stability. Clin Oral Implants Res. 2004. 15:520–528.
14. Berglundh T, Abrahamsson I, Lang NP, Lindhe J. DDe novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003. 14:251–262.
15. Misch CE. Density of bone: effect on treatment plans, surgical approach, healing, and progressive boen loading. Int J Oral Implantol. 1990. 6:23–31.
16. Albrektsson T, Jacobsson M. Bone-metal interface in osseointegration. J Prosthet Dent. 1987. 57:597–607.
17. Collier JP, Mayor MB, Chae JC, Surprenant VA, Surprenant HP, Dauphinais LA. Macroscopic and microscopic evidence of prosthetic fixation with porous-coated materials. Clin Orthop Relat Res. 1988. 235:173–180.
18. Pilliar RM. Porous-surfaced metallic implants for orthopedic applications. J Biomed Mater Res. 1987. 21:1–33.
19. Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001. 58:570–592.
20. Tal H, Dayan D. Spontaneous early exposure of submerged implants: II. Histopathology and histomorphometry of non-perforated mucosa covering submerged implants. J Periodontol. 2000. 71:1224–1230.
21. Uchida M, Kim HM, Kokubo T, Fujibayashi S, Nakamura T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J Biomed Mater Res A. 2003. 64:164–170.
22. Ducheyne P, Van Raemdonck W, Heughebaert JC, Heughebaert M. Structural analysis of hydroxyapatite coatings on titanium. Biomaterials. 1986. 7:97–103.
23. Cooley DR, Van Dellen AF, Burgess JO, Windeler AS. The advantages of coated titanium implants prepared by radiofrequency sputtering from hydroxyapatite. J Prosthet Dent. 1992. 67:93–100.
24. De Andrade MC, Sader MS, Filgueiras MR, Ogasawara T. Microstructure of ceramic coating on titanium surface as a result of hydrothermal treatment. J Mater Sci Mater Med. 2000. 11:751–755.
25. Kim HM, Miyaji F, Kokubo T, Nakamura T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J Biomed Mater Res. 1996. 32:409–417.
26. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000. 21:1803–1810.
27. Webster TJ, Schadler LS, Siegel RW, Bizios R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 2001. 7:291–301.
28. Oh SH, Finõnes RR, Daraio C, Chen LH, Jin S. Growth of nanoscale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials. 2005. 26:4938–4943.
29. Karlsson M, Pålsgård E, Wilshaw PR, Di Silvio L. Initial in vitro interaction of osteoblasts with nano-porous alumina. Biomaterials. 2003. 24:3039–3046.
30. Derhami K, Wolfaardt JF, Faulkner G, Grace M. Assessment of the periotest device in baseline mobility measurements of craniofacial implants. Int J Oral Maxillofac Implants. 1995. 10:221–229.




 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download