Abstract
STATEMENT OF PROBLEM
Despite an improved bone reactions of Mg-incorporated implants in the animals, little yet has been carried out by the experimental investigations in functional loading conditions.
PURPOSE
This study investigated the clinical and histologic parameters of osseointegrated Mg-incorporated implants in early loading conditions.
MATERIAL AND METHODS
A total of 36 solid screw implants (diameter 3.75 mm, length 10 mm) were placed in the mandibles of 6 beagle dogs. Test groups included 18 Mg-incorporated implants. Turned titanium implants served as control. Gold crowns were inserted 4 weeks after implant placement and the dogs were immediately put on a food diet. Implants were observed for 10 weeks after loading. Radiographic assessments and stability tests were performed at the time of fixture installation, 2nd stage surgery, 4 weeks after loading, and 10 weeks after loading. Histological observations and morphometrical measurements were also performed.
RESULTS
Of 36 implants, 33 displayed no discernible mobility, corresponding to successful clinical function. There was no statistically significant difference between test implants and controls in marginal bone levels (P = .46) and RFA values. The mean BIC% in the Mg-implants was 54.5 ± 8.4%. The mean BIC% in the turned implant was 45.3 ± 12.2%. These differences between the Mg-implant and control implant were statistically significant (P = .005).
It has been advocated that after implant placement, surgical sites should be undisturbed for at least 3-6 months to allow uneventful wound healing, thereby enhancing osseointegration between the implant and bone.1 However, the reduction of the postsurgical interval between implant placement and implant loading has been a crucial part of dental implantology.2
Early loading was regarded as being unpredictable. It might induce micromotions at the bone-implant interface that may lead to a fibrous encapsulation instead of direct bone apposition. Fibrous encapsulation around implant was a common finding due to a variety of reasons such as poor implant materials, designs, lack of understanding the mechanical aspect of implant loading, and etc.3,4 Since the resistance of the micromotion in the bone-implant interface depends on the initial mechanical fixation between the implant and the bone, it is likely to have high micromotion and stress gradients around the neck of implants might be caused by early loading.5 This may exceed the physiological tolerance threshold of bone. Indeed, excessive micromotion is directly implicated in the formation of fibrous encapsulation. The literature suggests that there is a critical threshold of micromotion above which fibrous encapsulation prevails over osseointegration. This critical level, however, is not zero micromotion as generally interpreted. Instead, the tolerated micromotion threshold was found to lie somewhere between 50 and 150 µm.6
Recently, the results on the early and immediate loading of single-tooth implants has been reported.7 It can be estimated that survival rate of single-tooth implants was high. In an animal study, the bone response for early-loaded implants was relatively favorable.8 The bone within the threads of the implant was highly mineralized and inflammatory cells were not found. These histological observations along with the clinical results suggest that early and immediate loading of implants might be successfully applied.9
Implant configuration and surface modification has long been considered as an essential requirement for implant success, especially, in challenging situations such as early and immediate loading conditions.2,10 As a general concept, the thread designs were developed for higher mechanical retention, greater stress distribution, and increased surface area.11 The thread design can minimize micromotion of the implant and improve the initial stability, which is the principal requirement for the success of early loading.
Implant surface characteristics may influence the bone response during the healing period in presence of the micromovements. Two action mechanisms of osseointegration by the surface modification have been proposed, which are biomechanical interlocking and biochemical bonding. Surface innovations for clinical implants have been attempted to enhance the biomechanical interlocking, particularly on topographical changes.
Potential chemical bonding between implant and host tissues was first suggested by Hench et al.12 and referred then to a certain glass-ceramic composition and its reaction to the host tissues. Although substantial experiments were performed, bioglass ceramics did not become commonly used for oral implants. Calcium phosphate (eg, hydroxyapatite) was regarded as potentially bioactive surface coatings for titanium implants. Calcium phosphate has similarities to bone mineral. They may form bone apatite like mineral or carbonate HA on their surfaces. They are able to promote cellular function, leading to formation of a strong bone-calcium phosphate interface. In addition, they are osteoconductive and may bind bone morphogenetic proteins (BMP) to become osteoinductive.
Recently, the implant systems having new surface were developed on the basis of anodic oxidation methods. This implant system has more than 2 µm thick oxides prepared by anodic oxidation. Anodic oxidation processes have been extensively investigated by several authors due to the potential of controlling the oxide properties. Ishizawa and coworkers13 developed unique methods of anodic oxidation with the electrolyte containing Ca and P. Oxidized surface was characterized by porous oxide and a tubular structure inter-channeled between pores. These implants showed significantly higher pushout strengths at 4, 8 and 24 weeks of the healing time in rabbit bone model.
Most recently, significance of surface chemistry of Calcium (Ca)-incorporated, Sulfur (S)-incorporated, Phosphate (P)-incorporated, Mg-incorporated implants to bone tissue reactions has been reported.14-16 These were anodized in electrolytes containing calcium, sulphur, phosphorus, and magnesium ions. These techniques were the surface chemistry modification of the implant that incorporated specific ions in the titanium oxide layer. These specific ions seem to be capable of reinforcing the bone-bonding as compared to implants consisted mainly of titanium oxide.
Among these specific ion-incorporated implants, Mg-implant has been studied in the most recent due to its biological possibilities. Mg-implant showed significantly stronger integration in bone compared to the oxidized implant that consisted mainly of TiO2. Sul et al.16 reported that the enhanced bone responses may not be explained by mechanical interlocking via bone growth into pores, but probably related to the surface oxide chemistry of the Mg-incorporated oxidized implants. Bonding failure generally occurred at the bone to implant interface for the machined-turned implant and mainly occurred in the bone away from the interface for the Mg-implant. Between bone and the Mg-incorporated implant surface, it was proposed that the ionic movements and ion concentrations gradient took place. They concluded that biochemical bonding may occur faster than micropore-mediated mechanical interlocking in rabbit bone and magnesium incorporated implants showed the significant improvement of implant stability and bonding strength.
Several hypotheses about the roles of Mg have been proposed. Matin and Brown17 investigated magnesium's role during synthetic hydroxyapatite formation in vitro and possible related effects during biomineralization. Mg2+ ion was more effective in promoting the binding of α1β1 integrin to collagen IV. Zreiqat et al.18 found increased adhesion of human bone-derived cells to Mg2+. Lapidos et al.19 reported Mg2+ acted as a potent chemoattractant. However, exact mechanism of inducing bone response has not been disclosed.
Despite improved bone reactions of Mg-incorporated implants in the animals, little yet has been carried out by the experimental investigations in functional loading conditions. Therefore, further animal and clinical studies are needed for clinical use of this implant.
The purpose of this study was to evaluate the clinical and histologic parameters of osseointegrated Mg-incorporated implants in early loading conditions.
Total 36 titanium implants (ASTM Grade IV) with a length of 10 mm, an outer diameter of 3.75 mm, a pitch height of 0.6 mm, and a turned surface were used. In this study, turned titanium screws were used as a control group. Electrochemically oxidized implants incorporating Mg (Mg-implants) in the surface titanium oxide layer were test group (Fig. 1). The Mg-implants were prepared using the micro arc oxidation (MAO) process at high forming voltages and current densities at galvanostatic mode in a magnesium containing mixed electrolyte system.
Chemical surface analysis was investigated using XPS (ESCALAP 250, VG Scientific Ltd). The XPS spectra were recorded using normal Al Kα radiation (1486.8 eV) with probing beam size of 200 µm. The outmost surface of implants was etched with Ar ion with ion energy of 5 KeV and beam current of 0.3 µA for 150 seconds, corresponding to 2 nm in thickness, resulting in removal of surface contaminants. The surface oxides of turned-implants and Mg-implants were consisted mainly of TiO2. XPS survey spectrum of the Mg-implants revealed the presence of the magnesium elements, Ti, O, C and some traces like P and S (Fig. 2a). The relative atomic concentration of Mg was approximately 7.6% at the as-received surface and 9.3% after Ar+ sputter cleaning for 150 seconds. 15% of C at the as-received surface was rapidly decreased after sputter cleaning. This may indicate the C is a surface contaminant. The mean surface porosity was 23.7%. The pore size was ≤ 1.5 µm in diameter. The pore density (pore population/scanning area) was 3.25. The mean oxide thickness was 3.4 µm in test Mg-implants as measured with a scanning electron microscope (S-3000N; Hitachi Science System, Tokyo, Japan) (Fig. 2b) on cross-section view and 17 nm in controls as measured with Auger Electron Spectroscopy (AES). The surface roughness measured with Optical Interferometer (MicroXam™) revealed 0.68 (± 0.2) µm Sa.
Six male beagle dogs, 24 months of age and 13 kg weight, were selected. All subjects were healthy and had no periodontal disease.
Bilateral mandibular premolars were extracted under general anesthesia. The anesthesia protocol for all surgical and follow-up procedures included premedication with acepromazine IM (Sedaject; Samu Median Co., Yesan, Korea), followed by 1:1 ratio mixtures of ketamine hydrochloride (Yuhan-Ketamine; Yuhan Co., Seoul, Korea) and xylazine hydrochloride (Rompun; Bayer Animal Health Co., Seoul, Korea). Initial dosage was 5 cc, and additional dosage 3 cc for maintaining anesthesia. Local infiltration was also performed with 2% lidocaine (Lidocaine HCl; Huons Co., Seoul, Korea).
After a full thickness mucoperiosteal flaps were raised adjacent to the mandibular premolars, teeth were hemisected under copious irrigation with a small fissure bur and disc. Extraction was performed with elevators and forceps. Flaps were closed with single interrupted 4-0 Gore-Tex sutures (Gore-Tex® Suture; W. L. Gore & Associates, Inc., Newark, USA).
Postoperative care protocol included antibiotics (Kanamycin-100; Samyang Pharma-Chem. Co., Seoul, Korea) and pain control (Hipyrine; Samyang Pharma-Chem. Co., Seoul, Korea) for 5 days.
Three months after extractions, surgical placement of implants was performed under sterile conditions. After adequate anesthesia, an incision was made through the mucosa at the crest of the alveolar ridge and a full thickness mucoperiosteal flaps were elevated in each mandibular quadrant over the healed extraction sites. Three implant sites were prepared per mandibular quadrant. Implant sites were gradually drilled with a round bur, 2.0 mm twist drill, pilot drill, and 2.85 mm twist drill (Nobel Biocare, Göteborg, Sweden). During surgical sequence, low rotary speed and profuse saline irrigation were used. The implants were inserted with a custom-made fixture mount via a connector and finally tightened manually with a screwdriver. A distance of approximately 7 mm between implant centers was maintained. The implants were placed in such a way that the fixture margin coincided with the bone crest. Cover screws were connected and the mucoperiosteal flaps were repositioned with mattress and single interrupted 4-0 Gore-Tex sutures. Each beagle dog received 3 control and 3 test implants which were inserted in the left and right side, respectively.
After 4 weeks, the surgical site was reopened for healing abutment connection and impression taking. Non-rotational fixture level impression copings (Neoplant® Neobiotech, Seoul, Korea) were connected to implants and secured with screws. Impression copings on each side of the mandible were splinted together using Duralay pattern resin (DuraLay; Reliance Dental Mfg Co., Worth, USA). Polyether impression material (Impregum; ESPE Co., MN, USA) was used for pick-up impression. After healing abutment connection, flap closure was accomplished with single interrupted 4-0 Gore-Tex sutures, leaving the healing cap exposed.
Fixture analogs were connected to the impression copings. Master casts were poured in type IV dental stone (Die-Keen® Heraeus Kulzer, Inc. New York, USA). Individual crowns were waxed on the abutments to proper contacts and contours. Total 36 single crowns were invested (Investment; Whipmix Co., Louisville, USA) and cast in type III gold (DM 76; Wooridongmyung Co., Seoul, Korea) (Fig. 3a). After devesting, extraoral occlusal adjustments were performed.
In 2 days after impression, the gold crowns were placed on the implants and intraoral occlusal adjustment performed to eliminate any direct occlusal contact on closure in premolar areas. All lateral occlusal interferences were removed. Gold crowns were subsequently connected to the abutments by means of abutment screws with a torque of 20 Ncm (Fig. 3b).
During the study period, three times weekly tooth brushing with a soft brush and 0.2% chlorhexidine gel (USP, Maryland, USA) cleaning were performed. For 2 weeks after each surgery, chlorhexidine swabbing of the surgical wounds was performed instead of tooth brushing to minimize mechanical trauma. In addition, for the first 2 weeks after surgery, the animals received a soft diet in an attempt to reduce mechanical trauma that could negatively influence healing process.
During the experiment, the several parameters were measured: (1) radiographic evaluation of peri-implant radiolucency, (2) measurement of marginal bone levels and calculation of changes over time, and (3) implant stability as measured with resonance frequency analysis (RFA, Osstell; Integration Diagnostics Ltd., Göteborg, Sweden).
For the radiographic interpretations, standardized periapical radiographs were used. The radiographs were taken with a customized occlusal index fabricated by affixing a siloxane putty impression material. For obtaining a parallel image, individual extension cone paralleling (XCP) device was made (Fig. 4). Standardized exposure parameters and processing procedures were applied. Each radiograph was then analyzed for changes in bone levels. Bone level changes were evaluated mesial and distal to each implant measuring the distance in millimeters between the top of implant head and the most coronal point of direct bone-to-implant contact.
RFA measurements were carried out by screwing a transducer (Type F1L5; Integration Diagnostics Ltd., Göteborg, Sweden) to the top of the fixture. Measurements were repeated on each implant by rotating the transducer and orienting it perpendicular and parallel to the long axis of the mandible.
Comparisons were made between implant stability quotient (ISQ) values obtained at the time of fixture installation, 2nd stage surgery, 4 weeks after loading, and 10 weeks after loading.
At the time of 10 weeks after loading, all subjects were sacrificed with an overdose of sodium-pentothal and perfused with a fixative through the carotid arteries. Implants and their surrounding tissues were removed en bloc, and immersed in 10% neutral buffered formalin (Accustain® Sigma-Aldrich®, Inc., Steinheim, Germany) for 1 week. After dehydration in a graded series of ethanols, the specimens were infiltrated, and embedded in methyl methacrylate (Technovit® 7200 VLC-resin, Kulzer, Friedrichsdorf, Germany) for nondecalcified sectioning according to Donath's protocol.20 Transverse sections, 100 µm in thickness, were taken along the long axis of the fixture by using a band saw with a diamond blade (Exakt Appratebau, Norderstedt, Germany), representing the mid portion of each implant site. Each section was ground to approximately 20 µm or less utilizing an EXAKT Micro Grinder, and polished to an optical finish. The specimens were stained with 1% toluidine blue.
A gross histological observations and morphometrical measurements were performed. Leitz Aristoplan™ microscope (Leitz, Wetzlar, GmbH, Germany) and Microvid™ equipment connected to an IBM computer were used. Measurements were carried out directly in the eye-piece of the light microscope using a 10× magnification objective and a zoom of 2.5×. The morphometric measurements were performed blindly. The mean percentages of bone-to-implant contact (BIC) and bone area in each thread (left and right side) were calculated. New bone formation around the implant within the threads was regarded as an R area. Mirror images (S area) of the R areas were also evaluated for status of original bone remodeling (Fig. 5). Marginal bone level as evaluated from the distance between the top of the implant head and the most coronal part of direct bone-to-implant contact.
Associations between the implant type and resulting data were evaluated by analysis of covariance (ANOVA) with repeated measurements. For histomorphometric analysis, the Mann-Whitney U test for comparison in-between implants was performed. And Wilcoxon signed rank test were used for paired comparisons (R area Vs S area) within each animal. With Mann-Whitney U test as well as Wilcoxson signed-rank test, two sided test was used and P ≤ .05 was considered statistically significant.
One implant in turned-implant at 4 weeks after fixture installation and two Mg-implants after 4 weeks of loading were lost due to unknown inflammation. No other implants showed peri-implant radiolucencies throughout the study. Overall survival rate was 91.7%.
The changes in marginal bone levels as determined radiographically are presented in Fig. 6. An increase in the bone level indicated bone loss around the implant. Both groups showed gradual bone loss. Comparing the bone levels at baseline and 10 weeks after loading, a statistically significant differences were found according to time lapse (P = .000). Overall bone loss was 1.5 mm in the control group and 1.3 mm in the Mg-implant group. However, there was no statistically significant difference between test implants and controls (P = .46).
The RFA values varied as a function of time. Initial mean ISQ value was 60.5 in the control group and 60.8 in the Mg-implant group (Fig. 7). Mean ISQ was slightly decreased in the control group at second stage surgery. However, these values were almost same at the 10 weeks after loading (62.9 Vs. 62.8 for the control group and Mg-implant group, respectively). The statistical analysis showed that the differences between the implants at implant installation and at 10 weeks after loading (P = .000). However, repeated measured ANOVA could not demonstrate the statistically significant difference between Mg-implants and controls through overall measuring time.
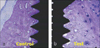
Bone shape, quantity and quality varied the most prominently in the anterior installation site. Implants of both groups were surrounded by mature bone within the threads and in direct contact with the implant surface. Intimate bone-implant contact was established, although it was occasionally interrupted by soft tissue. Bone filled almost the entire area within the threads of the control implants (Fig. 8a). Mg-implants had a thin bone collar in contact with their surfaces, whereas the areas within the threads were not occupied completely by bone (Fig. 8b). No signs of inflammatory reaction were detected in the tissues surrounding the implants.
The mean BIC% in the Mg-implants was 54.5 ± 8.4%. The mean BIC% in the turned implant was 45.3 ± 12.2%. These differences between the Mg-implant and control implant were statistically significant (P = .005) (Table I). Mean BIC in anterior installation site regardless of implant types was 48.1%. These were 46.1% and 39.3% in middle and posterior installation site, respectively. The mean BIC decreased from the anterior site to the posterior site. However, these data did not show a significant difference (P = .35).
The results from histomophometric analysis in bone area are presented in Table II. New bone formation in control implants was 72.8 ± 7.3% (R1 area), and 56.8 ± 8.5% (R2 area) in Mg-implants (Table II). Comparing the bone area inside the threads, there was significantly more bone within the threads in the control group than in the Mg-implant group (P = .000). The bone area in S1 (original bone remodeling in control implants) showed a significantly higher than in S2 (Mg-implants) (P = .023).
First bone contact of Mg-implants was 0.69 ± 0.47 mm, whereas 0.80 ± 0.35 mm in turned implants. However, these data were not statistically significantly different between Mg-implants and controls (P = .817). First bone contact in sectioned specimens was shorter than the data from radiographic measurements.
The present study showed a possibility of early loading after the implant placement. One of the turned implants failed, and two of the Mg-implants failed. Failure could be related to the primary implant stabilization, as recorded by the RFA. All failing implants were ranked 50 (ISQ value) and below. Loading was also seemed to influence the result, considering two failed Mg-implants after 4 weeks of loading placed the most distal area.
We used the stability and histomorphometric analysis for evaluating the early loaded implant. For standard parallel images, individual XCP was fabricated using a customized index. Each radiograph was computer digitized and analyzed for changes in marginal bone levels. The accuracy of this method was determined to be 0.2 mm in a repeated measurement. This interpretation of periapical radiographs enabled the observation of bone level changes during the 10-week loading period. It was suggested the high marginal bone loss to be a specific feature of early loading protocols. Akagawa et al.21 and Sagara et al.22 compared early loaded implants with non-loaded those in preclinical models. Although not quantified, both studies reported higher marginal bone loss for the early loaded group after 3 months of loading. In our study, an average bone loss was 1.5 mm in turned implant and 1.3 mm in Mg-implant during the observation period of 14 weeks. These results might be somewhat higher bone loss if it was compared to the non-loading condition. It would be related to the amount of occlusal loading. The occlusion of the beagle dogs in this study was a functional loading mode without direct occlusal contacts. Therefore, the restorations with full occlusal contact would be expected to produce a greater load.
RFA is well-documented biomechanical techniques that evaluate the stability of the osseointegrated implant.23 Clinical tests have revealed that the RFA values are related to the height of the implant that is not surrounded by bone, and to the interfacial stiffness.24,25 Several factors we found no significant differences in RFA values, between turned implant and Mg-implant due to several factors. Both implants had the same macro-design and similar surface roughness (0.55 µm in turned implant and 0.68 µm in Mg-implant). These results were similar to the study by Larsson et al.26
Bone response from deduction of BIC in loading conditions can be increased if the implant has a topographically changed surfaces i.e. hydroxyapatite-coated,27 sandblasted,28 etched,29 and etched-sandblasted.30 However, there were few studies on relations between BIC and surface chemistry in loading conditions. It is generally believed that the degree of BIC varies depending on implant macro/micro structures, surface characteristics, different healing period, and the presence or absence of loading.31,32
In the present study, test Mg-implants had a significantly more BIC than control turned implants. This may be meant that Mg-implants are much more effective in osseointegration in early loading conditions. Various surface oxide properties may influence the bone tissue response in this study, i.e. oxide thickness, surface roughness, and chemical composition. The oxide thicknesses in the present study were 17 nm in turned implant and 3.4 µm in Mg-implant. Increasing the oxide thickness of commercially pure titanium implants from about 3 to 200 nm in thickness resulted in no significant effects on the overall bone response as deduced from other study.33 In general, increasing the oxide thickness and resultant transition of the crystal structure (to anatase and rutile) is known to result in high corrosion resistance, which in turn favours excellent biocompatibility. 34 McAlarney et al.35 reported that in vitro C3 adsorption to anatase and rutile structures in thermally created oxides increased with increasing oxide thickness. Fujibayashi et al.36 reported that after sodium removal of alkali-and heattreated titanium, a faster bone-bonding was achieved due to its anatase surface structure. However, Li37 reported that bone showed a similar response to c.p. titanium and titania (rutile) in push out tests performed in a rabbit femoral model at 1 and 3 months after insertion.
After all, differing chemical properties between Mg-implants and turned implants, surface magnesium chemistry may be the most possible explanation for the improved BIC. XPS spectrum of the Mg-implants revealed the presence of the Mg elements, P and S comparing with turned implant. Considering that chemical bonding play an important role in initial healing phase, we could expect superior bone response of Mg-implants. Future studies are needed to understand the magnesium's role in initial healing phase.
Mg-implant was thought to induce more inflammation due to bioactive chemical composition. However, the results with no significant differences in histomorphometric first bone-to-implant contact and marginal bone loss represent that chemical modifications do not induce the inflammation.
The results of the present study indicate the possibility of rein-forced osseointegration being dependent on both biochemical bonding and mechanical interlocking focusing on the bone-to-implant contact. The biochemical bonding occurs rapidly and its stronger than mechanical interlocking alone. The faster and stronger osseointegration may have attractive clinical advantages as follows: 1) Higher success rate of implants in poor bone quality as well as in good bone quality may ensure. 2) Reduction of the bone healing time from implant installation to functional load bearing may be possible.
Several limitations were associated with this study. We didn't select a comparison group, such as other roughened surface or other ion-incorporated surface. The present study did not give solid evidence of biochemical bonding. It is extremely difficult to prove biochemical bonding in vivo due to complex dynamics at the bone to implant interface. Further investigations are needed focusing on the roles of the Mg-implant and also for the better understanding of what type of chemical bonding taking place. There is an unavoidable evolution and rush for early and immediate loading of implants, which has an important impact on the psycho-social well-being of patients. To obtain high success with early and immediate loaded implants, it is essential to increase our knowledge on bone response, especially, biochemical interaction around loaded implants. Therefore, fundamental studies are needed to elucidate the mechanisms responsible for functional adaptation of bone to implants subjected to various loading regimens in order to control or avoid bone loss around the loaded implants, and to provide predictable results for early and immediate implants in man.
The effect of early loading condition applied to Mg-implants and turned implants was evaluated clinically and histomorphometrically. On the basis of the results of this study, the following conclusions were drawn.
The anodized, Mg-incorporated implant demonstrated significantly more bone-to-implant contact (BIC) in early loading conditions. This experimental data may provide positive evidences for the surface chemistry-mediated biochemical bonding theory of bioactive implants. However, this study does not rule out potential synergy effects of the oxide thickness, micro-porous structure, and surface roughness on improvement of bone responses to Mg-implants.
Figures and Tables
References
1. Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977. 16:1–132.
2. Randow K, Ericsson I, Nilner K, Petersson A, Glantz PO. Immediate functional loading of Brånemark dental implants. An 18-month clinical follow-up study. Clin Oral Implants Res. 1999. 10:8–15.
3. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986. 1:11–25.
4. Albrektsson T, Sennerby L. State of the art in oral implants. J Clin Periodontol. 1991. 18:474–481.
5. Brunski JB. Avoid pitfalls of overloading and micromotion of intraosseous implants. Dent Implantol Update. 1993. 4:77–81.
6. Szmukler-Moncler S, Salama H, Reingewirtz Y, Dubruille JH. Timing of loading and effect of micromotion on bone-dental implant interface: review of experimental literature. J Biomed Mater Res. 1998. 43:192–203.
7. Gomes A, Lozada JL, Caplanis N, Kleinman A. Immediate loading of a single hydroxyapatite-coated threaded root form implant: a clinical report. J Oral Implantol. 1998. 24:159–166.
8. Piattelli A, Corigliano M, Scarano A, Quaranta M. Bone reactions to early occlusal loading of two-stage titanium plasma-sprayed implants: a pilot study in monkeys. Int J Periodontics Restorative Dent. 1997. 17:162–169.
9. Piattelli A, Corigliano M, Scarano A. Microscopical observations of the osseous responses in early loaded human titanium implants: a report of two cases. Biomaterials. 1996. 17:1333–1337.
10. Lefkove MD, Beals RP. Immediate loading of cylinder implants with overdentures in the mandibular symphysis: the titanium plasma-sprayed screw technique. J Oral Implantol. 1990. 16:265–271.
11. Misch CE. Implant design considerations for the posterior regions of the mouth. Implant Dent. 1999. 8:376–386.
12. Hench LL, Paschall HA. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J Biomed Mater Res. 1973. 7:25–42.
13. Ishizawa H, Ogino M. Formation and characterization of anodic titanium oxide films containing Ca and P. J Biomed Mater Res. 1995. 29:65–72.
14. Sul YT, Johansson CB, Kang Y, Jeon DG, Albrektsson T. Bone reactions to oxidized titanium implants with electrochemical anion sulphuric acid and phosphoric acid incorporation. Clin Implant Dent Relat Res. 2002. 4:78–87.
15. Sul YT, Byon ES, Jeong Y. Biomechanical measurements of calcium-incorporated oxidized implants in rabbit bone: effect of calcium surface chemistry of a novel implant. Clin Implant Dent Relat Res. 2004. 6:101–110.
16. Sul YT, Johansson C, Byon E, Albrektsson T. The bone response of oxidized bioactive and non-bioactive titanium implants. Biomaterials. 2005. 26:6720–6730.
17. Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995. 29:389–401.
18. Zreiqat H, Howlett CR, Zannettino A, Evans P, Schulze-Tanzil G, Knabe C, Shakibaei M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J Biomed Mater Res. 2002. 62:175–184.
19. Lapidos KA, Woodhouse EC, Kohn EC, Masiero L. Mg(++)-induced endothelial cell migration: substratum selectivity and receptor-involvement. Angiogenesis. 2001. 4:21–28.
20. Donath K. . Preparation of histologic sections by cutting-grinding technique for hard tissue and other materials not suitable to be sectioned by routine methods. 1993. Norderstedt: EXAKT-Kulzer-Publication;1–6.
21. Akagawa Y, Ichikawa Y, Nikai H, Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993. 69:599–604.
22. Sagara M, Akagawa Y, Nikai H, Tsuru H. The effects of early occlusal loading on one-stage titanium alloy implants in beagle dogs: a pilot study. J Prosthet Dent. 1993. 69:281–288.
23. Meredith N, Shagaldi F, Alleyne D, Sennerby L, Cawley P. The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clin Oral Implants Res. 1997. 8:234–243.
24. Friberg B, Sennerby L, Linden B, Gröndahl K, Lekholm U. Stability measurements of one-stage Brånemark implants during healing in mandibles. A clinical resonance frequency analysis study. Int J Oral Maxillofac Surg. 1999. 28:266–272.
25. O'Sullivan D, Sennerby L, Meredith N. Measurements comparing the initial stability of five designs of dental implants: a human cadaver study. Clin Implant Dent Relat Res. 2000. 2:85–92.
26. Larsson C, Thomsen P, Lausmaa J, Rodahl M, Kasemo B, Ericson LE. Bone response to surface modified titanium implants: studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials. 1994. 15:1062–1074.
27. de Lange GL, Donath K. Interface between bone tissue and implants of solid hydroxyapatite or hydroxyapatite-coated titanium implants. Biomaterials. 1989. 10:121–125.
28. Buser D, Nydegger T, Hirt HP, Cochran DL, Nolte LP. Removal torque values of titanium implants in the maxilla of miniature pigs. Int J Oral Maxillofac Implants. 1998. 13:611–619.
29. Davies JE. In vitro modeling of the bone/implant interface. Anat Rec. 1996. 245:426–445.
30. Wong M, Eulenberger J, Schenk R, Hunziker E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J Biomed Mater Res. 1995. 29:1567–1575.
31. Cochran DL. The scientific basis for and clinical experiences with Straumann implants including the ITI Dental Implant System: a consensus report. Clin Oral Implants Res. 2000. 11:33–58.
32. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991. 25:889–902.
33. Larsson C, Thomsen P, Lausmaa J, Rodahl M, Kasemo B, Ericson LE. Bone response to surface modified titanium implants: studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials. 1994. 15:1062–1074.
34. Chen G, Wen X, Zhang N. Corrosion resistance and ion dissolution of titanium with different surface microroughness. Biomed Mater Eng. 1998. 8:61–74.
35. McAlarney ME, Oshiro MA, McAlarney CV. Effects of titanium dioxide passive film crystal structure, thickness, and crystallinity on C3 adsorption. Int J Oral Maxillofac Implants. 1996. 11:73–80.
36. Fujibayashi S, Nakamura T, Nishiguchi S, Tamura J, Uchida M, Kim HM, Kokubo T. Bioactive titanium: effect of sodium removal on the bone-bonding ability of bioactive titanium prepared by alkali and heat treatment. J Biomed Mater Res. 2001. 56:562–570.
37. Li J. Behaviour of titanium and titania-based ceramics in vitro and in vivo. Biomaterials. 1993. 14:229–232.




 PDF
PDF ePub
ePub Citation
Citation Print
Print











 XML Download
XML Download