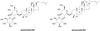This article has been retracted. See "Withdrawal: Protective effects of cultured and fermented ginseng extracts against scopolamine-induced memory loss in a mouse model" in Volume 34 on page 329.
Abstract
This study was performed to investigate the effect of a concentrate of fermented wild ginseng root culture (HLJG0701) on memory improvement in the scopolamine (SPL)-induced memory-deficient mouse model. Eight-week-old male ICR mice were used to evaluate the protective effect of HLJG0701 against the SPL-induced memory loss animal model. The Morris water maze test, which measures hippocampus-dependent learning ability, and the Y-maze test, a short-term memory assessment test, were performed and related markers were analyzed. HLJG0701-treated groups displayed significantly reduced acetylcholinesterase activity and increased acetylcholine level compared with the SPL-administered group (SPL-G) (P<0.05). In the Y-maze test, the spontaneous alternation in al HLJG0711-treated groups was significantly increased compared with that in SPL-G (P<0.05). In the Morris water maze test, the escape latency and time spent in the target quadrant in all HLJG0701-treated groups were significantly decreased and increased, respectively, compared with those in SPL-G (P<0.05). In addition, the brain-derived neurotrophic factor level in groups treated with HLJG0701 300 and 600 mg/kg body weight was significantly increased compared with that in SPL-G (P<0.05). These results suggest that the HLJG0701 may protect against memory loss by inhibiting acetylcholinesterase activity and preventing acetylcholine deficiency.
Memory refers to the ability to remember or reconstruct an experience or information with the hippocampus of the brain playing a crucial role [1]. In the rapidly changing modern society, the importance of memory, which is recognized as an important factor in terms of quality of life, continues to increase, especially in the elderly [2]. The most common factor of memory deficit in the elderly is Alzheimer's disease (AD), accounting for 60–70% of all demented cases [3]. Most of the original pathologic research on neurodegeneration of the cholinergic system was performed on AD [4]. Substantial loss of cholinergic innervation in the cerebral cortex is universally accepted as an aspect of advanced AD [5].
Scopolamine (SPL), a muscarinic cholinergic receptor antagonist, inhibits the function of cholinergic neurotransmitters when administered to experimental animals, inducing short-term memory loss through the inhibition of acetylcholine (ACh) [6]. Therefore, SPL has often been used in animal models to develop medical products or health functional foods to improve memory [7].
Wild ginseng (Panax ginseng C.A Mayer) refers to ginseng found in mountain regions that has a superior efficacy to cultivated ginseng, although they look similar. It has been difficult for the public to use wild ginseng because of unavailability and high prices. To resolve these problems, wild ginseng root culture methods have been studied using tissues isolated from wild ginsengs [8]. A previous study examined the effects of ginsenosides on memory improvement [9].
The pharmacological activities of ginseng are mainly attributed to ginsenosides [10], which are composed of a dammarane skeleton (17 carbons in a four-ring structure) with various sugar moieties attached to the C-3 and C-20 positions [11]. Fermentation of ginseng extract allows controlled bioconversion of ginsenosides to more bioavailable and bioactive metabolites [12]. Research has shown that the transformation of ginsenosides into deglycosylated ginsenosides Rk1 and Rg5 during fermentation is required in order for them to provide more effective in vivo physiological action [13].
Up to the present day, various studies have been conducted to prevent and treat neurological diseases such as dementia, which damage memory and learning abilities, resulting in the production of an ACh precursor, receptor agonist, and acetylcholinesterase (AChE) inhibitor [1415]. Nevertheless, there is a growing need for the development of new preventive and treatment agents for neurological disorders because of the unsatisfactory efficacies and accompanying side effects of available treatments [16]. Thus, this study evaluated whether a concentrate of fermented wild ginseng root culture (HLJG0701) containing various ginsenosides at high concentrations, affected memory improvement in SPL-induced memory-deficient animal models.
The SPL, AChE activity assay kit, and choline/ACh quantification kit were purchased from Sigma Chem. Co. (St. Louis, MO, USA). The brain-derived neurotrophic factor (BDNF) enzyme-linked immunosorbent assay (ELISA) kit was purchased from Abcam (Cambridge, MA, USA).
The original wild ginseng (Panax ginseng C. A. Meyer) was obtained from Cheon-Ill Korean Pharmacy (Yanggu, Korea) and used for in vitro culture. After high-temperature and high-pressure treatments, the cultured wild ginseng roots (CWG) were dried using hot air to adjust the water content to below 5%, which was followed by concentration under low pressure, resulting in a Brix of 60. The suspension was prepared by the addition of 12-fold purified water (w/w) into the concentrate of CWG, and then a lactic acid bacterial strain was inoculated for fermentation. After fermentation, the culture was deactivated and concentrated, and a concentrate of HLJG0701 was obtained. The levels of ginsenosides Rg5 and Rk1 in HLJG0701 were 21.4 and 12.3 mg/g, respectively (Figure 1).
Eight-week-old male ICR mice were purchased from Orient Bio (Seongnam, Korea). All animals were acclimated to the laboratory environment for 7 days. Two animals were housed per cage, allowed access to water and food ad libitum, and maintained in a constant temperature (23±2℃), humidity (55±10%), air change (12 times/hour) and illumination intensity (150–300 Lux) environment under a 12-h light/dark cycle (light period, 08:00–20:00 h). This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Hoseo Toxicological Research Center (HTRC) (HTRC IACUC 16-97).
Animals were divided in the following group (8 animals each): normal control (CON), one group treated with SPL 1.0 mg/kg body weight (BW) (SPL-G), three groups treated with different doses of HLJG0701 (150 mg/kg BW (HG-150), 300 mg/kg BW (HG-300), 600 mg/kg BW (HG-600)) and one group treated with 10 mg/kg BW of tetrahydroaminoacridine (THA-10) which has recently been shown to improve memory and cognitive functions in some patients with Alzheimer's disease [17]. Animals were dosed via gastric intubation 6 times a week for 5 weeks.
After completion of the 5-week treatment, 1 mg/kg of SPL was injected intraperitoneally to all animals except the CON 30 min prior to behavior assessment. For the 5 days of behavior assessment, all groups were given the drug specific to their groups 4 h prior to behavior assessment.
At the end of the administration and behavioral test, all animals were autopsied after euthanasia, and brain tissues were sampled and stored at −70℃ until further analysis.
Brain tissues were weighed and mixed with 0.01 M phosphate-buffered saline (PBS) in a 1:7 (w/v) ratio. After homogenization and centrifugation at 8000 rpm for 15 min, the supernatant was obtained. The supernatant was used to measure AChE activity, ACh content, and BDNF levels.
After completion of the behavioral test, brain tissues collected from animals were homogenized to measure AChE activity, and ACh and BDNF content. According to the instruction manuals, these values were determined using an AChE activity assay kit, ACh quantification kit and BDNF ELISA kit, respectively.
The water maze test was adapted from the paradigm originally described by Morris [18]. The water maze was a circular pool (diameter 90 cm, height 55 cm), filled with water to a depth of 30 cm, and a platform was placed approximately 1 cm below the water surface. Spatial cues were attached to one side of the wall to adhere to the standard.
To provide experimental animals with a standard reference point, a spatial cue was fixed to the wall. To allow the animals to adapt to swimming, they were given 1 min of free swimming without a platform from 2 days before the beginning of the trial. In the main tests, SPL (1 mg/kg BW) was abdominally administered 30 min before the first trial. The maze was divided into 4 zones, and the animals were allowed to swim in the zones without a platform. One swimming session lasted for 60 sec, and was repeated 3 times at 10 min intervals. When the animals climbed onto the platform, they were allowed to stay for 20 sec, and if they were unable to find the spatial cue within 60 sec, they were placed on the platform for 20 sec to let them identify the spatial cue. The experiment was repeated for 5 days, and the time it took for the mice onto climb the platform was measured. On the sixth day, the platform was removed, and the experiment was performed for 60 sec in the same manner to measure the time spent in the zone where the platform used to be.
SPL was administered by the same method as in the water maze test. The Y-maze test is a horizontal maze with three black arms (A, B and C; 42 cm-long and 3 cm-wide with 12 cm-high walls) that are symmetrically disposed at 120 angles from each other. The test was performed as previously described [19] the day after the water maze test was completed. The animals were put on one arm of the Y-maze and allowed to move freely for 8 min, and then the total number of entries into each of arms A, B and C and the order in which the animals entered the arms were recorded. Using these results, the ratio of alternation behavior was calculated according to the following equation: Alternation behavior (%)=Actual alternation/(Total entry−2)×100 (Actual alternation: 1 point was given when an animal entered into the different arms in order).
The data were presented as the mean±standard deviation (SD). The statistical significance of the data was tested by analysis of variance (ANOVA) using SPSS (Version 12.0, SPSS Inc., Chicago, IL, USA) followed by Tukey's post-hoc test. For a single comparison, the significance of differences was determined by the t-test. The data were presented as the mean±standard deviation (SD). Statistical significance was set at P<0.05.
To identify the effect of HLJG0701 on memory improvement in SPL-induced memory-deficient animals, AChE activity and ACh content in the brain tissue were measured (Figure 2). The AChE activity in SPL-G was significantly increased compared with that in CON (P<0.05). In all HLJG0701-treated groups, AChE activity decreased compared with that in SPL-G. However, no significant difference was observed based on the dose of the test substance. The AChE activity in THA-10 was significantly decreased compared with that in all HLJG0701-treated groups (P<0.05) and significantly increased compared with that in CON (P<0.05) (Figure 2A). ACh content in all HLJG0701-administered groups was significantly increased compared with that in SPL-G (P<0.05), but significantly decreased compared with that in CON (P<0.05). In addition, ACh content in THA-10 was significantly increased compared with that in SPL-G and all HLJG0701-administered groups (P<0.05) (Figure 2B).
In the SPL-induced memory-deficient animals, the Y-maze test was performed to examine the effect of HLJG0701 on short-term memory improvement. The alternation behavior in all HLJG0701-treated groups was significantly increased compared with that in SPL-G (P<0.05), and that in THA-10 meaningfully increased compared with SPL-G (P<0.05) (Figure 3B). In the HLJG0701-administered groups, no distinctive difference was found in the total entry numbers among the groups, suggesting that the alternation behavior results were not attributable to enhanced activity (Figure 3A). On the other hand, there was no significant difference in the total entry numbers between SPL-G and all HLJG0701-treated groups.
To investigate the effect of HLJG0701 on BDNF expression in the SPL-induced memory-deficient animals, the hippocampus tissue was used for quantitative analysis because the hippocampus is one of the first regions of the brain to suffer damage in Alzheimer's disease and other forms of dementia [20]. No significant difference was found in the BDNF level between CON and SPL-G. However, the BDNF level in HG-300, HG-600 and THA-10 was significantly increased compared with SPL-G (P<0.05) (Figure 4).
The Morris water maze test was performed to confirm the effect of HLJG0701 on spatial learning ability in SPL-induced memory-impaired mice. Until the 3rd day post-treatment, the experimental groups did not show any statistical significance for the time taken to climb the platform. However, the escape latency time in SPL-G on the 4th and 5th day post-treatment was meaningfully increased compared with that in all HLJG0701-treated groups (4th day, P<0.05; 5th day, P<0.01), CON and THA-10 (P<0.001) (Figure 5A). On the 6th day after the Morris water maze test, the time spent in the target quadrant for SPL-G was significantly decreased compard with the corresponding CON, THA-10 and all HLJG0701-administered groups (P<0.05). The time spent in the target quadrant for HG-150 and HG-300 was significantly increased compared with that for CON (P<0.05). However, there was no significant difference in the time spent in the target quadrant between CON, HG-600 and THA-10 (Figure 5B).
The brain exhibits complex interactions of neural systems to maintain its function. The cholinergic system is known to play the most important role in memory [221], and ACh is its most crucial neurotransmitter. ACh is synthesized from choline and acetyl-CoA by acetyl-transferase, which is in turn catalyzed into acetate and choline by AChE. It is known that when AChE activity is enhanced, the ACh level decreases, leading to memory loss [22]. Therefore, if a substance can either inhibit AChE activity or increase the ACh level, memory loss can be prevented [23].
This study was carried out to evaluate the preventive effect of HLJG0701 on memory loss using memory loss animal models induced by SPL, which blocks the cholinergic system. The present study found that HLJG0701 decreased AChE activity and increased the level of ACh. These results are consistent with the findings of previous studies using ginsenoside Rg1 [24], ginsenoside Rg3-enriched ginseng extract [25] and Panax ginseng root alcohol extract [26]. In a previous study, contents of ginsenoside Rb1, Rc, Rd, Rf, Rg1 and Rh1 in wild ginseng were higher than those in cultured ginseng 4 and 6 years, and only trace amounts of ginsenoside Rg3 were contained in wild ginseng [27]. In addition, another study reported that fermented wild ginseng root cultures did not change the total saponin content or ginsenoside profile compared to wild ginseng root cultures, but improved functionality [13].
BDNF is a major neurotrophic factor that regulates neuronal cell growth. BDNF not only promotes the production of acetylcholine by increasing its expression in the hippocampus, but also enhances synaptic plasticity; thus, it was speculated that there is a close relationship between BDNF and memory [28]. In this study, the level of BDNF in HG-300 and HG-600 meaningfully increased compared with that in SPL-G, indicating that HLJG0701 at concentrations of 300 and 600mg/kg has an ameliorating effect on scopolamine-induced memory-impaired mice. In a previous study [29], BDNF expression in the brain of mice treated with an alcoholic extract of Ashwagandha leaves (100, 200 and 300 mg/kg BW) for 7 days, was significantly increased compared with that in mice treated with SPL alone (P<0.05). In another previous study [30], the BDNF expression level in the hippocampus of 12-month-old mice administered with ginsenoside (0.028% (P<0.05), 0.056% (P<0.05) and 0.112% (P<0.01), w/v) via drinking water for 3 months, was meaningfully increased compared with the control administered with normal water. Considering the dosage and treatment period, the increased BDNF level of HLJG0701 used in this study was higher than that of the ginsenoside and lower than that of the alcoholic extract of Ashwagandha leaves.
In a previous study on spontaneous alternation behavior using a Y-maze test [31], 20~21-month-old mice administered with red ginseng extract (200 mg/kg/day) for 3 months displayed significantly enhanced spontaneous alteration compared with mice treated with saline. However, no difference was found on the total entry numbers between red ginseng-treated mice and saline-treated mice. In addition, Dela Peña et al. [32] reported that in mice pretreated with Vietnamese ginseng methanol extract (20 (P<0.05), 50 (P<0.01) and 100 (P<0.001) mg/kg) and Korean red ginseng pure extract (100 mg/kg, P<0.001) for 1 day or 7 days, spontaneous alternation significantly increased compared with that in mice treated with SPL (1.5 mg/kg), but there were no significant differences in total entry numbers between the ginseng-treated groups and the SPL-treated group.
In a previous Morris water maze study on mice [29], the escape latency in mice treated with white (P<0.001), red (P<0.01) and black (P<0.01) ginseng extracts (200 mg/kg BW) on day 4 was significantly lower than that in mice treated with SPL. Furthermore, the time in the target quadrant in all ginseng-treated groups on day 5 was meaningfully deceased compared with that in the group treated with SPL (2 mg/kg BW) (P<0.001). In another previous study [33], the escape latency in the group treated with ginsenoside Rg3 (20 and 40 mg/kg BW, P<0.05) and ginseol k-g3 (50 (P<0.01), 100 and 200 (P<0.05) mg/kg BW) on day 5, was meaningfully decreased compared with that in the SPL-administered group. In addition, the time in the target quadrant in the group treated with ginsenoside Rg3 (40 mg/kg BW, P<0.001) and ginseol k-g3 (25, 50, 100 and 200 mg/kg BW, P<0.01) on day 6 was significantly increased compared with that of the SPL-treated group. In reducing the escape latency and increasing the time in target quadrant, HLJGO701 used in this study was more effective than white, red and black ginseng extracts, but less effective than ginsenoside Rg3 and ginseol k-g3.
In conclusion, the results from this study suggest that HLJG0701 can protect against SPL-induced impairment of spatial learning ability and memory in mice by inhibiting AChE activity and preventing Ach deficiency. Further investigation into behavioral and biochemical methods is inquired to clarify the exact mechanism of action of HLJG0701 for future studies.
Figures and Tables
 | Figure 2Acetylcholinesterase activity (A) and acetylcholine content (B) in the brain tissues of mice treated with saline (control, CON), scopolamine (1 mg/kg, i.p., SPL-G), HLJG0701 (150 mg/kg, HG-150; 300 mg/kg, HG-300; 600 mg/kg, HG-600, p.o.) and tetrahydroaminoacridine (10 mg/kg, p.o., THA-10) 6 times per week for 5 weeks. Data are expressed as the mean±SD (n=8). Means with different superscripts are significantly different (P<0.05). |
 | Figure 3Effect of HLJG0701 on scopolamine-induced memory deficit in the Y-maze test. Mice in different groups were administered with saline (control, CON), scopolamine (1 mg/kg, i.p., SPL-G), HLJG0701 (150 mg/kg, HG-150; 300 mg/kg, HG-300; 600 mg/kg, HG-600, p.o.) and tetrahydroaminoacridine (10 mg/kg, p.o., THA-10) 6 times per week for 5 weeks. The number of arm entries (A) and spontaneous alternation score (B) were recorded. Data are expressed as the mean±SD (n=8). Means with different superscripts are significantly different (P<0.05). |
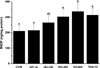 | Figure 4Effect of HLJG0701 on the increasing brain-derived neurotrophic factor (BDNF) content in murine brain tissues. Mice in different groups were administered with saline (control, CON), scopolamine (1 mg/kg, i.p., SPL-G), HLJG0701 (150 mg/kg, HG-150; 300 mg/kg, HG-300; 600 mg/kg, HG-600, p.o.) and tetrahydroaminoacridine (10 mg/kg, p.o., THA-10) 6 times per week for 5 weeks. The number of arm entries (A) and spontaneous alternation score (B) were recorded. Data are expressed as the mean±SD (n=8). Means with different superscripts are significantly different (P<0.05). |
 | Figure 5Effect of HLJG0701 on scopolamine-induced spatial memory impairment. (A) Escape latency to find the platform during the training stages. (B) The time spent in the target quadrant of the probe trial. Mice in different groups were administered with saline (control, CON), scopolamine (1 mg/kg, i.p., SPL-G), HLJG0701 (150 mg/kg, HG-150; 300 mg/kg, HG-300; 600 mg/kg, HG-600, p.o.) and tetrahydroaminoacridine (10 mg/kg, p.o., THA-10) 6 times per week for 5 weeks. Training trial sessions were conducted for 5 days and retention times were measured on day 6. Data are expressed as the mean±SD (n=8). Means with different superscripts are significantly different (P<0.05). |
References
1. Monti JM, Baym CL, Cohen NJ. Identifying and characterizing the effects of nutrition on hippocampal memory. Adv Nutr. 2014; 5(3):337S–343S.

2. Oh SK. Neurotransmitters and brain disease. Seoul: Shinil Books Company;2005. p. 345–364.
3. Fratiglioni L, Winblad B, von Strauss E. Prevention of Alzheimer's disease and dementia. Major findings from the Kungsholmen Project. Physiol Behav. 2007; 92(1-2):98–104.

4. Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011; 221(2):564–573.

5. Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011; 221(2):555–563.

6. Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010; 34(8):1307–1350.

7. Oh JH, Choi BJ, Chang MS, Park SK. Nelumbo nucifera semen extract improves memory in rats with scopolamine-induced amnesia through the induction of choline acetyltransferase expression. Neurosci Lett. 2009; 461(1):41–44.

8. Han JY, Chung KH, Ryu GH. Comparison of physicochemical properties and release characteristics of extruded tissue cultured mountain ginseng. J Korean Soc Food Sci Nutr. 2008; 37(8):1018–1024.

9. Zhu G, Wang Y, Li J, Wang J. Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience. 2015; 292:81–89.

10. Niu J, Pi ZF, Yue H, Yang H, Wang Y, Yu Q, Liu SY. Effect of 20(S)-ginsenoside Rg3 on streptozotocin-induced experimental type 2 diabetic rats: A urinary metabonomics study by rapid-resolution liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2012; 26(23):2683–2689.

12. Bai Y, Gänzle MG. Conversion of ginsenosides by Lactobacillus plantarum studied by liquid chromatography coupled to quadrupole trap mass spectrometry. Food Res Int. 2015; 76(Pt 3):709–718.
13. Shin EJ, Cho CW, Kim YE, Han D, Hong HD, Rhee YK. Evaluation of functional properties of the tissue cultured wild ginseng fermented by Lactobacillus sp. Korean J Food Cult. 2012; 27(6):743–750.
14. Panza F, Lozupone M, Solfrizzi V, Stallone R, Bellomo A, Greco A, Daniele A, Seripa D, Logroscino G. Cognitive frailty: a potential target for secondary prevention of dementia. Expert Opin Drug Metab Toxicol. 2017; 13(10):1023–1027.

15. Rakesh G, Szabo ST, Alexopoulos GS, Zannas AS. Strategies for dementia prevention: latest evidence and implications. Ther Adv Chronic Dis. 2017; 8(8-9):121–136.

16. Wittstock M, Zettl UK. Adverse effects of treatment with intravenous immunoglobulins for neurological diseases. J Neurol. 2006; 253:Suppl 5. V75–V79.

17. Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J. A controlled trial of tacrine in Alzheimer's disease. JAMA. 1992; 268(18):2523–2529.

18. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11(1):47–60.

19. Kim DH, Jeon SJ, Son KH, Jung JW, Lee S, Yoon BH, Lee JJ, Cho YW, Cheong JH, Ko KH, Ryu JH. The ameliorating effect of oroxylin A on scopolamine-induced memory impairment in mice. Neurobiol Learn Mem. 2007; 87(4):536–546.

21. Terry AV Jr, Mahadik SP. Time-dependent cognitive deficits associated with first and second generation antipsychotics: cholinergic dysregulation as a potential mechanism. J Pharmacol Exp Ther. 2007; 320(3):961–968.

22. Remya C, Dileep KV, Tintu I, Variyar EJ, Sadasivan C. Flavanone glycosides as acetylcholinesterase inhibitors: computational and experimental evidence. Indian J Pharm Sci. 2014; 76(6):567–570.
23. Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasiæ VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013; 11(3):315–335.
24. Wang Q, Sun LH, Jia W, Liu XM, Dang HX, Mai WL, Wang N, Steinmetz A, Wang YQ, Xu CL. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother Res. 2010; 24(12):1748–1754.

25. Kim J, Shim J, Lee S, Cho WH, Hong E, Lee JH, Han JS, Lee HJ, Lee KW. Rg3-enriched ginseng extract ameliorates scopolamine-induced learning deficits in mice. BMC Complement Altern Med. 2016; 16:66.

26. Al-Hazmi MA, Rawi SM, Arafa NM, Wagas A, Montasser AO. The potent effects of ginseng root extract and memantine on cognitive dysfunction in male albino rats. Toxicol Ind Health. 2015; 31(6):494–509.

27. Jeong HS, Lim CS, Cha BC, Choi SH, Kwon KR. Component analysis of cultivated ginseng, cultivated wild ginseng, and wild ginseng and the change of ginsenoside components in the process of red ginseng. J Pharmacopuncture. 2010; 13(1):63–77.

28. Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009; 15(3):331–337.

29. Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, Thakur MK. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011; 6(11):e27265.

30. Zhao HF, Li Q, Li Y. Long-term ginsenoside administration prevents memory loss in aged female C57BL/6J mice by modulating the redox status and up-regulating the plasticity-related proteins in hippocampus. Neuroscience. 2011; 183:189–202.

31. Lee Y, Oh S. Administration of red ginseng ameliorates memory decline in aged mice. J Ginseng Res. 2015; 39(3):250–256.

32. Dela Peña IJI, Kim HJ, Botanas CJ, de la Peña JB, Van Le TH, Nguyen MD, Park JH, Cheong JH. The psychopharmacological activities of Vietnamese ginseng in mice: characterization of its psychomotor, sedative-hypnotic, antistress, anxiolytic, and cognitive effects. J Ginseng Res. 2017; 41(2):201–208.