Abstract
Diabetes is a major risk factor for stroke and is also associated with worsened outcomes following a stroke. Peroxiredoxin-2 exerts potent neuroprotective effects against oxidative stress. In the present study, we identified altered peroxiredoxin-2 expression in an ischemic stroke model under hyperglycemic conditions. Adult male rats were administrated streptozotocin (40 mg/kg) via intraperitoneal injection to induce diabetes. Middle cerebral artery occlusion (MCAO) was induced surgically 4 weeks after streptozotocin treatment and cerebral cortex tissues were isolated 24 hours after MCAO. Peroxiredoxin-2 expression was evaluated in the cerebral cortex of MCAO-operated animals using a proteomics approach, and was found to be decreased. In addition, the reduction in peroxiredoxin-2 levels was more severe in cerebral ischemia with diabetes compared to animals without diabetes. Reverse-transcriptase PCR and Western blot analyses confirmed the significantly reduced peroxiredoxin-2 expression in MCAO-operated animals under hyperglycemic conditions. It is an accepted fact that peroxiredoxin-2 has antioxidative activity against ischemic injury. Thus, the findings of this study suggest that a more severe reduction in peroxiredoxin-2 under hyperglycemic conditions leads to worsened brain damage during cerebral ischemia with diabetes.
Stroke has both a high incidence and mortality. Among patients who experience a stroke, cerebral ischemia can lead to serious disabilities due to neurological deficits and complications. The neurological dysfunction following cerebral ischemia is due to a massive loss of neurons. Moreover, diabetes mellitus increases the risk of mortality after ischemic stroke and is associated with worsened functional recovery [1]. As an explanation for this association, hyperglycemia increases oxidative stress and the activity of matrix metalloproteinase-9 as well as exacerbating blood-brain barrier disruption and formation of edema [2]. Likewise, hyperglycemia leads to vascular complications and increased incidence of cerebrovascular disease [3]. Hyperglycemia aggravates central nervous system damage caused by cerebral ischemia.
Oxidative stress leads to impaired mitochondrial function of neurons and subsequent cell death. Oxidative stress is well-appreciated as a major contributor to the initiation of diabetic complications and the progression of diabetic neuropathy [4]. Hyperglycemia is associated with increased susceptibility to oxidative stress in cerebral ischemia and more severe brain damage. Specifically, hyperglycemia increases superoxide production and promotes hemorrhage [5], as well as increases the generation of ROS and contributes to the progression of neurovascular disease [56].
Peroxiredoxins are ubiquitous family proteins that act as antioxidant enzymes by converting various peroxides such as H2O2 and hydroperoxides to water and alcohol, respectively. Peroxiredoxins comprise six subtypes with various cellular functions including cell proliferation, gene expression, differentiation, and apoptosis [7]. Likewise, peroxiredoxins maintain redox balance under both oxidative stress and normal conditions [8910]. Peroxiredoxin-2 is one of six isoenzymes containing redox-active cysteines capable of reducing peroxides. Peroxiredoxin-2 is abundantly expressed in brain tissue and is considered to be neuron specific. In addition, peroxiredoxin-2 is involved in various neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, and brain ischemia [1112]. Overexpression of peroxiredoxin-2 protects cortical neurons against apoptosis by attenuating the apoptosis signal-regulating kinase signaling cascade [1314]. Peroxiredoxin-2 also has a protective effect against oxidative stress in neuronal cell culture and decreases brain damage after transient brain ischemia [1215]. We previously reported that peroxiredoxin-2 is significantly decreased in focal cerebral ischemic damage [16]. Moreover, we previously showed that diabetes mellitus is associated with increased infarct volume and exacerbation of brain damage during focal cerebral ischemia [17]. Based on these data, we hypothesized that preoxiredoxin-2 expression may be more significantly decreased in focal cerebral ischemia under hyperglycemic conditions compared to normoglycemic conditions. Thus, in the present study, we investigated whether hyperglycemia induced by streptozotocin affected peroxiredoxin-2 expression during cerebral ischemic injury.
Forty male Sprague-Dawley rats (200-220 g, 9 weeks old) were obtained from Samtako Co. (Animal Breeding Center, Osan, Korea) and were randomly allocated to four groups: non-diabetic+sham, diabetic+sham, non-diabetic+middle cerebral artery occlusion (MCAO), and diabetic+MCAO. Animals were allowed free access to food and water under controlled room temperature (25℃) and lighting (14:10 light/dark cycle) conditions for this study. All procedures were approved by the ethics committee concerning animal research at Gyeongsang National University (GNU-141223-R0063) and were performed in accordance with the committee guidelines.
Diabetes was induced by a single intraperitoneal injection of streptozotocin (Sigma, St. Louis, MO, USA) at a dose of 40 mg/kg [1819]. Streptozotocin was freshly prepared in 10 mM citrate buffer (pH 4.6), and non-diabetic control animals were injected with citrate buffer only. Blood glucose concentrations were monitored using an Accu-Chek® Active (Roche Diagnostics GmbH, Mannheim, Germany) glucose meter and diabetes mellitus was defined as hyperglycemic conditions with a glucose concentration exceeding 300 mg/dL. The body weight was decreased dose- and time dependent after streptozotocin injection [2021].
MCAO surgery is the most common stroke model and was performed as previously described method 4 weeks after streptozotocin injection [22]. Body weight and blood glucose were measured shortly before the MCAO operation. Animals were anesthetized with Zoletil (Virbac, Carros, France, 50 mg/kg) by intramuscular injection and placed on an operation plate in the supine position. After a midline neck incision, the precervical fascia and muscle were dissociated. The right common carotid artery and the right external carotid artery were then exposed. Next, the proximal part of the right common carotid artery was temporarily blocked with microvascular clips and the external carotid artery was ligated and cut. The pterygopalatine artery was also dissociated and ligated. A 4/0 monofilament nylon suture, the tip of which was rounded by heating, was then inserted through the external carotid artery and advanced into the internal carotid artery, thus occluding the origin of the middle cerebral artery. The external carotid artery was tightly ligated with monofilament nylon and the microvascular clip on the right common carotid artery was carefully removed. A sham operation was performed using the same procedure described above but without insertion of the monofilament. At 24 h after the onset of MCAO, the animals were decapitated and the brain tissues were isolated. Brain tissues were frozen in liquid nitrogen and stored at −70℃ for analysis. For the determination of infarct volume, the brains were quickly removed and cut into 2 mm thick coronal slices using a rat brain matrix, reacted with 2% 2,3,5-triphenyltetrazolium chloride (TTC) solution at 37℃ for 10 min, and then fixed with 10% formalin. The stained images were scanned using an Agfa ARCUS 1200™ (Agfa-Gevaert, Mortsel, Belgium) and infarct volumes were analyzed using Image-ProPlus 4.0 software (Media Cybernetics, Silver Spring, MD, USA). The volume of each infarct was determined as a percentage of the infarction area relative to the whole slice area.
A proteomic technique was used as described previously [23]. Briefly, samples were homogenized in lysis buffer (8 M urea, 4% CHAPS, ampholytes, and 40 mM Tris-HCl) and centrifuged at 16,000 g for 20 min at 4℃. The resulting pellets were dissolved in lysis buffer and the concentration of protein was determined by a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). First-dimensional isoelectric focusing (IEF) was performed on an Ettan IPGphor 3 System (GE Healthcare, Uppsala, Sweden) and immobilized pH gradient (IPG) gel strips with a linear pH gradient from 4 to 7, and 6 to 9 (17 cm, Bio-Rad) were used. Samples were rehydrated in buffer (8 M urea, 2% CHAPS, 20 mM DTT, 0.5% IPG buffer, and bromophenol blue) for 13 h. The protein samples (100 µg) were then loaded onto IPG strips and IEF was performed as follows: 250 V for 15 min, 10,000 V for 3 h, and then 10,000 V to 50,000 V. Next, the strips were incubated in equilibration buffer [6 M urea, 30% glycerol, 2% SDS, 50 mM Tris-HCl (pH 8.8), 1% DTT] for 15 min. The strips were then applied to the top of gradient gels (7.5-17.5%) for the second dimension. Gels were electrophoresed using Protein-II XI electrophoresis equipment (Bio-Rad) at 5 mA for 2 hours and 10 mA for 10 hours at 10℃. After electrophoresis, the gels were incubated in fixation solution (12% acetic acid, 50% methanol) for 2 hours and rinsed with 50% ethanol for 20 min. The resulting gels were stained with silver solution (0.2% silver nitrate) for 20 min and then developed in a solution of 0.2% sodium carbonate. The stained gel images were obtained using an Agfa ARCUS 1200™ (Agfa-Gevaert) and image statistical analysis was performed using PDQuest 2-D analysis software (Bio-Rad). Protein spots were excised from the stained gels using a punch and processed for MALDI-TOF. The gel particles were digested in trypsin-containing buffer and then extracted. Tryptic peptides were mixed with a matrix solution and the resulting mixture was analyzed with a Voyager System DE-STR MALDI-TOF mass spectrometer (Applied Biosystem, Foster City, CA, USA) to obtain a peptide mass fingerprint. MS-Fit and ProFound software was used to detect proteins and the SWISS-Prot and NCBI databases were used to identify protein sequences.
Total RNA was isolated from frozen right cerebral cortical tissue samples using Trizol Reagent (Life Technologies, Rockville, MD, USA) according to the manufacturer's instructions. Purified RNA samples were stored at −70℃. RNA samples (1 µg) were converted into complementary DNA using the Superscript III first-strand system for reverse transcriptase-PCR (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. For PCR reactions, the amplification temperature and time were set as follows: 5 min at 94℃ followed by 30 cycles of 30 sec at 94℃, 30 sec at 54℃, and 1 min at 72℃, and finally 10 min at 72℃. PCR products were separated by 1% agarose gel electrophoresis and bands were visualized under ultraviolet light. The primer sequences for peroxiredoxin-2 were: 5'-AGGACTTCCGAAAGCTAGGC-3' (forward primer) and 5'-TTGACTGTGATCTGGCGAAG-3' (reverse primer). The primer sequences for actin were: 5'-AGGACTTCCGAAAGCTAGGC-3' (forward primer) and 5'-TTGACTGTGATCTGGCGAAG-3' (reverse primer).
Western blot analysis was performed according to a previously described method [23]. Frozen samples were rapidly homogenized in lysis buffer to which phenylmethanesulfonylfluoride was added as a protease inhibitor. Homogenates were sonicated to dissolve the tissue completely, centrifuged at 30,000 g for 30 min at 4℃, and the resulting supernatants were collected. Protein concentration was determined using a bicinchoninic acid kit (Pierce, Rockford, IL, USA) with albumin diluted in lysis buffer as the standard. Total protein (30 µg) from each sample was electrophoresed on 10% SDS-PAGE gels and completely transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) at 4℃. Membranes were blocked with 10% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 hour to minimize nonspecific antibody binding. The membranes were rinsed with TBST and incubated with the corresponding primary antibodies for 12 hours at 4℃. The primary antibodies used in this study were peroxiredoxin-2 and β-actin (diluted 1:1000, Cell Signaling Technology, Beverly, MA, USA). β-Actin was used as a control protein to verify equal protein loading. Membranes were rinsed with TBST and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000, Pierce) as a secondary antibody. Enhanced chemiluminescence reagent (GE Healthcare, Uppsala, Sweden) was used to detect signals according to the manufacturer's instructions. Intensity of Western blot images was measured with SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA).
All data are expressed as the mean±SEM. Intensity analysis was performed using SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, USA). The results for each group were compared with two-way analysis of variance (ANOVA) followed by a post-hoc Scheffe's test. Differences were considered statistically significant at P<0.05.
We detected an obvious difference in body weight and blood glucose in diabetic animals treated with streptozotocin compared to control animals, with diabetic animals exhibiting decreased body weight and increased blood glucose levels. Specifically, body weights were 312.7±23.5 g and 197.5±18.5 g in non-diabetic animals and diabetic animals, respectively (Supplementary Figure 1). Blood glucose levels were 102.5±3.5 and 352.5±28.4 mg/dL in non-diabetic animals and diabetic animals. After performing the MCAO operation, we found that infarct volume was significantly increased in diabetic animals compared to non-diabetic animals. Specifically, infarct volume was 27.45±3.25 and 38.65±4.25% in non-diabetic+MCAO animals and diabetic+MCAO animals, respectively.
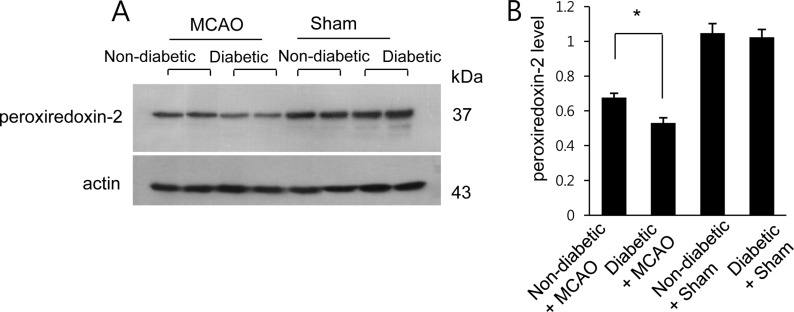
A proteomics-based approach identified a decrease in peroxiredoxin-2 expression in MCAO-operated animals. The peptide mass of peroxiredoxin-2 was 9/51 and the sequence coverage was 46%. Diabetic animals exhibited a more severe decrease in peroxiredoxin-2 expression compared to non-diabetic animals after MCAO injury; however, peroxiredoxin-2 expression was similar in non-diabetic and diabetic animals that underwent the sham operation (Figure 1A). On the other hand, when we evaluated peroxiredoxin-2 levels as a ratio to the intensity of non-diabetic+sham for non-diabetic+MCAO animals and diabetic+MCAO animals, the resulting values were 0.72±0.03 and 0.65±0.05, respectively (Figure 1B). We next confirmed the reduction in peroxiredoxin-2 expression in MCAO-operated animals by reverse transcriptase-PCR and Western blots analyses. Consistent with our proteomics approach, diabetic animals with MCAO injury exhibited a more severe decrease in peroxiredoxin-2 expression compared to non-diabetic animals. Specifically, the normalized transcript levels of peroxiredoxin-2 were 0.50±0.03 and 0.18±0.04 in non-diabetic+MCAO animals and diabetic+MCAO animals, respectively (Figure 2). Likewise, peroxiredoxin-2 protein levels were 0.68±0.04 in the cerebral cortices of non-diabetic+MCAO animals and 0.53±0.03 in diabetic+MCAO animals (Figure 3).
Hyperglycemia is associated with more severe brain damage from focal cerebral ischemia compared to normal conditions. Consistently, diabetes mellitus exacerbates neuronal apoptosis and necrosis in the cerebral cortex of cerebral ischemic animals [24]. In the present study, we confirmed the presence of decreased body weight and hyperglycemia in animals treated with streptozotocin prior to MCAO surgery, which resulted in increased brain tissue damage in these rats compared to normoglycemic rats. The results of this study are consistent with the results of previous studies showing that hyperglycemia is associated with increased ischemic infarct size and worse outcomes following cerebral ischemia compared to normoglycemia conditions [2425].
Peroxiredoxin-2 attenuates neuronal cell death in brain ischemic injury through its antioxidant capacity, and overexpression of peroxiredoxin-2 significantly reduces brain injury and elevates neurological outcome in focal cerebral ischemia [13]. Peroxiredoxin-2 also has cytoprotective effects in cortical neuron primary culture under oxygen glucose deprivation [12]. We previously demonstrated that peroxiredoxin-2 expression is decreased in focal cerebral ischemia. As an extension of these findings, we identified a more severe decrease in the protein expression of peroxiredoxin-2 in cerebral ischemia under conditions of hyperglycemia. Reverse transcriptase-PCR and Western blot analyses were used to confirm the decrease in preoxiredoxin-2 in the cerebral cortex of animals with MCAO under diabetic conditions. Hyperglycemia leads to superoxide radical generation and the subsequent increase in oxidative stress results in increased cell death. Previous studies have shown that increased ROS levels are associated with decreased antioxidant capacity in diabetic subjects [2627]. The elevation of peroxiredoxin-2 expression through transfection transfection attenuates cell death from oxidative stress mediated apoptosis [28]. However, reduced levels of peroxiredoxin-2 exaggerated oxidative stress-induced apoptotic cell death [28]. Thus, it is considered that decrease of peroxiredoxin-2 leads to serious neurological disorder.
Streptozotocin is a toxic agent that induces hyperglycemic conditions through destruction of pancreatic β-cells. Streptozotocin also increases oxidative stress and causes tissue dysfunction [29]. Moreover, apoptosis induced by streptozotocin treatment is associated with significantly decreased peroxiredoxin-2 expression [28]. In the present study, we demonstrated that peroxiredoxin-2 was significantly decreased in cerebral ischemia in the presence of hyperglycemia compared to normoglycemia. Consistently, both hyperglycemia and ischemic conditions are known to potently increase the generation of oxidative radicals. Thus, the significant decrease of peroxiredoxin-2 and resulting loss of antioxidant capacity can cause worsened neurological deficits. Furthermore, diabetes exacerbates the decrease of peroxiredoxin-2 expression during cerebral ischemic injury. In conclusion, our findings provide evidence that severely decreased peroxiredoxin-2 exacerbates brain damage during cerebral ischemia in the diabetes setting.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST)(NRF-2015R1D1A1A01058270).
References
1. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001; 32(10):2426–2432. PMID: 11588337.
2. Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007; 38(3):1044–1049. PMID: 17272778.
3. Davidson JA, Parkin CG. Is hyperglycemia a causal factor in cardiovascular disease? Does proving this relationship really matter? Yes. Diabetes Care. 2009; 32(2):S331–S333. PMID: 19875575.
4. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011; 50(5):567–575. PMID: 21163346.

5. Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol. 2011; 70(4):583–590. PMID: 22002675.

6. Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013; 47(1):9–23. PMID: 23011809.

7. Kalinina EV, Chernov NN, Saprin AN. Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochemistry (Mosc). 2008; 73(13):1493–1510. PMID: 19216714.

8. Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999; 300:219–226. PMID: 9919524.

9. Chae HZ, Oubrahim H, Park JW, Rhee SG, Chock PB. Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators. Antioxid Redox Signal. 2012; 16(6):506–523. PMID: 22114845.

10. Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid Redox Signal. 2011; 15(3):831–844. PMID: 20919932.
11. Patenaude A, Murthy MR, Mirault ME. Emerging roles of thioredoxin cycle enzymes in the central nervous system. Cell Mol Life Sci. 2005; 62(10):1063–1680. PMID: 15761666.

12. Gan Y, Ji X, Hu X, Luo Y, Zhang L, Li P, Liu X, Yan F, Vosler P, Gao Y, Stetler RA, Chen J. Transgenic overexpression of peroxiredoxin-2 attenuates ischemic neuronal injury via suppression of a redox-sensitive pro-death signaling pathway. Antioxid Redox Signal. 2012; 17(5):719–732. PMID: 22356734.

13. Chen ML. Two-dimensional gel electrophoresis revealed antipsychotic drugs induced protein expression modulations in C6 glioma cells. Prog Neuropsychopharmacol Biol Psychiatry. 2013; 40:1–11. PMID: 22960606.

14. Hu X, Weng Z, Chu CT, Zhang L, Cao G, Gao Y, Signore A, Zhu J, Hastings T, Greenamyre JT, Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011; 31(1):247–261. PMID: 21209210.

15. Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res. 2007; 85(14):3089–3097. PMID: 17663478.

16. Koh PO. Proteomic analysis of focal cerebral ischemic injury in male rats. J Vet Med Sci. 2010; 72(2):181–185. PMID: 19942814.

17. Sung JH, Koh PO. Hyperglycemia aggravates decreases of PEA-15 and its two phosphorylated forms in cerebral ischemia. J Vet Med Sci. 2017; 79(3):654–660. PMID: 28216548.

18. Tancrede G, Rousseau-Migneron S, Nadeau A. Long-term changes in the diabetic state induced by different doses of streptozotocin in rats. Br J Exp Pathol. 1983; 64(2):117–123. PMID: 6221747.
19. Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, Ezquer F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther. 2016; 7:42. PMID: 26983784.

20. Oh TW, Kang SY, Park YK. Histological analysis of five organs in streptozotocin-induced diabetic rats. Korean J Herbol. 2013; 28(6):39–45.

21. Wang H, Li H, Jiang X, Shi W, Shen Z, Li M. Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes. 2014; 63(5):1506–1518. PMID: 24379355.

22. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91. PMID: 2643202.

23. Gim SA, Lee SR, Shah FA, Koh PO. Curcumin attenuates the middle cerebral artery occlusion-induced reduction in γ-enolase expression in an animal model. Lab Anim Res. 2015; 31(4):198–203. PMID: 26755923.

24. Rizk NN, Rafols J, Dunbar JC. Cerebral ischemia induced apoptosis and necrosis in normal and diabetic rats. Brain Res. 2005; 1053(1-2):1–9. PMID: 16038884.

25. Li ZG, Britton M, Sima AA, Dunbar JC. Diabetes enhances apoptosis induced by cerebral ischemia. Life Sci. 2004; 76(3):249–262. PMID: 15531378.

26. Haskins K, Bradley B, Powers K, Fadok V, Flores S, Ling X, Pugazhenthi S, Reusch J, Kench J. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003; 1005:43–54. PMID: 14679039.

27. Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002; 45(1):85–96. PMID: 11845227.

28. Zhao F, Wang Q. The protective effect of peroxiredoxin II on oxidative stress induced apoptosis in pancreatic β-cells. Cell Biosci. 2012; 2(1):22. PMID: 22709359.

29. Nukatsuka M, Sakurai H, Yoshimura Y, Nishida M, Kawada J. Enhancement by streptozotocin of O2- radical generation by the xanthine oxidase system of pancreatic beta-cells. FEBS Lett. 1988; 239(2):295–298. PMID: 2846360.
Supplementary material
Supplementary Figure 1
Blood glucose levels (A), body weight (B), and infarct volume (C) in non-diabetic+sham, diabetic+sham, non-diabetic+middle cerebral artery occlusion (MCAO), and diabetic+MCAO animals. Data (n=5) are shown as the mean±SEM. *P<0.01.
Figure 1
Proteomic analysis of peroxiredoxin 2 in the cerebral cortex of non-diabetic+sham, diabetic+sham, non-diabetic+middle cerebral artery occlusion (MCAO), and diabetic+MCAO animals. Circles indicate peroxiredoxin 2 protein spots. Mw and pI indicate molecular weight and isoelectrical point, respectively. Spot intensities were measured by PDQuest software. Spot intensities are represented as a ratio relative to non-diabetic+sham control animals. Data (n=5) are shown as the mean±SEM. *P<0.05.
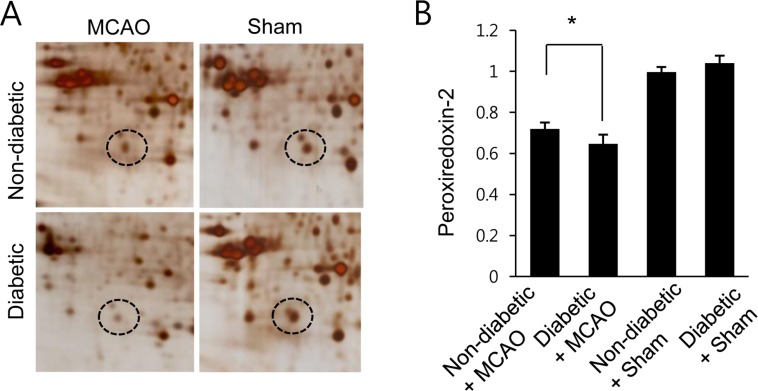
Figure 2
Reverse transcriptase-PCR of peroxiredoxin 2 in the cerebral cortex of non-diabetic+sham, diabetic+sham, non-diabetic+middle cerebral artery occlusion (MCAO), and diabetic+MCAO animals. Each lane represents an individual experimental animal. The band intensity of RT-PCR product was normalized to that of the actin product. Data (n=5) are shown as the mean±SEM. *P<0.05.
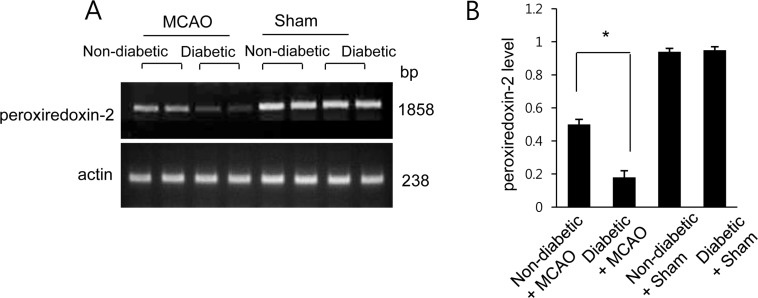
Figure 3
Western blot analysis of peroxiredoxin 2 in the cerebral cortex from non-diabetic+sham, diabetic+sham, non-diabetic+middle cerebral artery occlusion (MCAO), and diabetic+MCAO animals. Each lane represents an individual animal. Densitometric analysis is represented as a ratio of protein intensity to actin intensity. Data (n=5) are shown as the mean±SEM. *P<0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download