Abstract
Chronic obstructive pulmonary diseases (COPD) is an important disease featured as intense inflammation, protease imbalance, and air flow limitation and mainly induced by cigarette smoke (CS). In present study, we explored the effects of Pycnogenol® (PYC, pine bark extract) on pulmonary fibrosis caused by CS+lipopolysaccharide (LPS) exposure. Mice were treated with LPS intranasally on day 12 and 26, followed by CS exposure for 1 h/day (8 cigarettes per day) for 4 weeks. One hour before CS exposure, 10 and 20 mg/kg of PYC were administered by oral gavage for 4 weeks. PYC effectively reduced the number of inflammatory cells and proinflammatory mediators caused by CS+LPS exposure in bronchoalveolar lavage fluid. PYC inhibited the collagen deposition on lung tissue caused by CS+LPS exposure, as evidenced by Masson's trichrome stain. Furthermore, transforming growth factor-β1 (TGF-β1) expression and Smad family member 2/3 (Smad 2/3) phosphorylation were effectively suppressed by PYC treatment. PYC markedly reduced the collagen deposition caused by CS+LPS exposure, which was closely involved in TGF-β1/Smad 2/3 signaling, which is associated with pulmonary fibrotic change. These findings suggest that treatment with PYC could be a therapeutic strategy for controlling COPD progression.
Cigarette smoke (CS) is regarded as the major critical cause in development of chronic obstructive pulmonary disease (COPD) featured as an airway inflammation, emphysema, and small airway remodeling [1]. Small airway remodeling is variable and consist of a combination of symptoms such as pulmonary fibrosis and inflammatory cell infiltration [2]. In particular, pulmonary fibrosis is a crucial cause of mortality in patients with pulmonary disease including COPD since it is an irreversible alteration and reduces normal lung function [3]. Among various cytokines and growth factor, transforming growth factor-β1 (TGF-β1) is closely related to pulmonary fibrotic change [4]. In the development of pulmonary fibrosis, TGF-β1 plays crucial role in the production of the extracellular matrix, α-smooth muscle actin (α-SMA) and collagen via Smad signaling. A series of theses mechanisms eventually results in pulmonary fibrosis [5]. CS extract could modulate the TGF-β1/Smad pathway, and in clinical trials, alteration in TGF-β1/Smad signaling was observed in pulmonary fibrosis in patients with COPD [67]. Therefore, downregulation of TGF-β1/Smad is considered to be important in treating pulmonary fibrosis.
Pinus pinaster Aiton (French maritime pine) is used as traditional herbal medicine to enhance wound healing and to treat inflammatory disease in North America and Europe. Pycnogenol® (PYC, pine bark extract) is a trade name and a standardized mixture of the bark of French maritime pine [8]. Recent reports have demonstrated that PYC significantly suppresses inflammation, fat accumulation, and fibrosis in the liver and heart [910]. In addition, the preventive effect of PYC against wrinkle formation through TGF and type I procollagen has been described [11]. Accordingly, we hypothesized that PYC might effectively attenuates CS-induced pulmonary fibrosis. Despite the anti-fibrotic effect of PYC, its ability to protect against pulmonary fibrosis and underlying mechanisms has not been investigated previously.
Therefore, we have explored the effects of PYC affect on CS and lipopolysaccharide (LPS)-caused pulmonary fibrosis by measuring fibrotic mediators and performing histological analysis. Additionally, we further investigated the its protective mechanism on pulmonary fibrosis focusing on modulation of TGF-β1/Smad family member 2/3 (Smad 2/3) signaling.
C57BL/6N male mice (6-week-old, 20-25 g) were obtained from Koatech Co., (Pyeongtaek, Korea) and were provided sterilized tap water and commercial rodent chow (Samyang Feed Co., Wonju, Korea). All procedures were granted by the Institutional Animal Care and Use Committee of Chonnam National University (Gwangju, Korea).
CS exposure was performed as previously described [12]. LPS (10 µg/mouse, Sigma-Aldrich, MO, USA) was treated by intranasal instillation on day 12 and 26 under anesthesia. Roflumilast (Sigma-Aldrich) was a phosphodiesterase-4 inhibitor recommended for treating COPD and used as a positive control drug [13]. Roflumilast (10 mg/kg) and PYC (10 and 20 mg/kg, Horphag Research, Le Sen, France) were administered to animals for 4 weeks by oral gavage 1 h before the CS exposure. Tentative identification of phytochemicals in PYC was described in our previous study [12]. Briefly, five phytochemicals (procyanidin B-type dimer, procyanidin dimer gallate ester, procyanidin B-type trimer, taxifolin-3-O-β-glucoside, and taxifolin) were identified in PYC.
BALF was collected as previously described method [14]. Differential cell counts of BALF was conducted by Diff-Quik® reagent (Sysmex Corporation, Kobe, Japan). In quantitative analysis of Interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), we used commercial ELISA kit (BD Biosciences, CA, USA). The measurement of absorbance (450 nm) was conducted by spectrophotometer (Bio-Rad Lab., CA, USA).
Lung tissue process was performed in previous study [12]. Sectioned lung tissue was stained with hematoxylin and eosin (H&E, Sigma-Aldrich) to measure inflammatory responses and Masson's trichrome (Abcam, Cambridge, UK) to evaluate collagen deposition, according to the manufacturer's protocols.
Additionally, we have performed immunohistochemistry (IHC) to examine fibrosis-related protein expression as previously described [14]. The following antibodies were used: TGF-β1 (1:200; dilution, Abcam) and collagen (1:200 dilution; Santa Cruz Biotechnology, CA, USA)
Homogenization of lung tissue was performed using a tissue lysis/extraction reagent (Sigma-Aldrich) with a protease inhibitor cocktail (Sigma-Aldrich). Immunoblotting was performed as previously described [14]. The primary antibodies were used as follows; anti-collagen (1:1,000 dilution; Santa Cruz), anti-TGF-β1 (1:1,000 dilution; Abcam), anti-β-actin (1:2,000 dilution; Cell Signaling, MA, USA) anti-pSmad 2/3 (1:1,000 dilution; Abcam) and anti-Smad 2/3 (1:1,000 dilution; Abcam). Protein expression value was determined by ChemiDoc (Bio Rad Lab.).
Inflammatory cell counts including neutrophils, macrophages, and lymphocytes were remarkably elevated in CS and LPS exposed animals in comparison to normal controls (Figure 1). By contrast, the roflumilast-treated animals was observed the marked reduction in inflammatory cell counts in comparison to CS and LPS exposed animals. PYC-treated animals (10 and 20 mg/kg) significantly lower inflammatory cell counts than CS and LPS exposed animals, which was observed in dose-dependent manner.
The CS and LPS exposed animals showed a marked elevation of IL-1β level in comparison to normal controls (Figure 2A). However, roflumilast-treated animals meaningfully lower IL-1β level than CS and LPS exposed animals. PYC-treated animals were detected the notable decline of IL-1β level in comparison to CS and LPS exposed animals, which were observed in dose-dependent. Similarly, IL-6 and TNF-α levels were obviously elevated in CS and LPS exposed animals. However, roflumilast- and PYC-treated animals were observed the notable decline of IL-6 and TNF-α in comparison to CS and LPS exposed animals (Figure 2B and 2C).
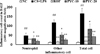
CS and LPS exposed animals exhibited the obvious inflammatory cell accumulation into peribronchiolar and perivascular lesions in comparison to normal controls (Figure 3). However, roflumilast- and PYC-treated animals were meaningfully decreased inflammatory cell accumulation in comparison to CS and LPS exposed animals.
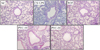
In lung tissue stained with Masson's trichrome, staining of collagen was only seen around vessels or bronchioles in normal controls (Figure 4). However, CS and LPS exposed animals exhibited the extensive collagen deposition in lung tissue. Roflumilast- and PYC-treated animals meaningfully reduced collagen deposition in comparison to CS and LPS exposed animals.
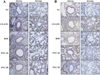
To evaluate the expression of TGF-β1 and collagen, we performed IHC in lung tissues of CS and LPS exposed animals. The CS and LPS exposed animals more increased TGF-β1 expression in lung tissue than normal controls (Figure 5A). However, roflumilast- and PYC-treated animals obviously decreased TGF-β1 expression in comparison to CS and LPS exposed animals. Similar to the results of TGF-β1, collagen I expression remarkably elevated in CS and LPS exposed animals in comparison to normal controls, and the increased expression levels were markedly reduced in the roflumilast- and PYC-treated animals (Figure 5B).
The CS and LPS exposed animals exhibited a notable elevation of TGF-β1 expression in comparison to normal controls (Figure 6). However, roflumilast- and PYC-treated animals showed significantly reduced expression of TGF-β1 in comparison to CS and LPS exposed animals. The phosphorylation of Smad 2/3 was markedly elevated in the CS and LPS exposed animals in comparison to normal controls. In contrast, roflumilast- and PYC-treated animals exhibited a marked suppression of the Smad 2/3 phosphorylation induced by CS+LPS exposure. The results of the analysis of collagen I expression were similar to those of TGF-β1 expression and Smad 2/3 phosphorylation. The CS and LPS exposed animals exhibited a significant elevation of collagen I expression in comparison to normal controls, whereas roflumilast- and PYC-treated animals showed significant decreases in comparison to CS and LPS exposed animals.
CS is regarded as a major threatening factor in the development of COPD [14]. CS induces not only extensive airway inflammation but also collagen deposition in lung tissue, resulting in pulmonary fibrosis [15]. In this experiment, we explored the effects of PYC on the inflammatory response and collagen deposition in CS and LPS exposed mice. In addition, we elucidated the molecular mechanisms of CS+LPS-induced pulmonary fibrotic changes. PYC markedly decreased inflammatory cell counts and inflammatory mediator induced by CS+LPS exposure. PYC also significantly suppressed the expression levels of TGF-β1 expression and Smad 2/3 phosphorylation, which resulted in reduced collagen deposition in lung tissue of the mice.
Inflammation is a key factor contributing lung disease and airflow limitation in COPD [16]. The characteristic pattern of inflammation in COPD animal models is an increased number of neutrophils and macrophages in the airway, lung parenchyma, and BALF [17]. These cells subsequently act as a crucial role in pathogenesis of chronic inflammation, resulting in the production of various cytokines and chemokines including TNF-α, IL-1β, and IL-6 [18]. Increased production of these inflammatory mediators amplifies not only additional inflammatory cell recruitment but also fibrotic responses representing collagen deposition [19]. It has been demonstrated that small airway fibrosis is a critical mechanism of disease progression in COPD patients and is closed associated with chronic inflammation [20]. In this experiment, PYC meaningfully reduced inflammatory cell accumulation and proinflammatory cytokines in BALF from the CS+LPS-exposed mice. These effects of PYC were consistent with those observed in previous studies [2122]. According to Shin et al. [23], PYC effectively inhibits airway inflammation in an allergen-induced asthma model. In particular, Xia et al. [24] demonstrated that PYC markedly decreased TNF-α, IL-1β, and IL-6 in BALF in ventilator-induced lung injury. Based on these observation, PYC was considered to possess protective effects in the CS+LPS animal model used in the present study.
Pulmonary fibrosis is a general term used to describe the increased accumulation of collagenous extracellular matrix, which compromises the expiratory airflow [25]. It is associated with various diseases including COPD [26]. In clinical trials, patients with COPD showed a marked increase in mesenchymal markers such as collagen I and α-SMA [3]. In particular, the small airways of patients with COPD characteristically accumulated mesenchymal cells and collagenous extracellular matrix [27]. CS is considered as a crucial factor in the development of COPD and induces fibrotic changes in the lung. Zhang et al. [28] reported that CS extract-stimulated cells showed elevated epithelial to mesenchymal transition. Additionally, CS contributed to lung remodeling represented by perinbronchiolar fibrosis in COPD [27]. In this study, the CS+LPS exposed mice showed increased collagen I expression compared with that of the normal controls based on histological evidence from lung tissue stained with Masson's trichrome and IHC for collagen I. However, the PYC-treated animals exerted a marked decreased in histological collagen deposition and collagen I expression in comparison to CS and LPS-exposed animals. It has been demonstrated that PYC significantly suppresses fat accumulation and fibrosis in the liver of high-fat diet model and prevents the development of viral myocarditis by decreasing inflammation and fibrosis [910]. These findings indicate that PYC may suppress the fibrotic change in the lung tissue caused by CS and LPS exposure.
In this study, CS+LPS-exposed animals showed the activation of TGF-β1/Smad 2/3 signaling, which resulted in increased collagen I expression. However, PYC effectively suppressed the activated TGF-β1/Smad 2/3 signaling accompanied with decreased collagen I expression. TGF-β1 plays a crucial role in development of pulmonary fibrosis, and TGF-β1/Smad signaling is the most thoroughly explored potential underlying mechanism [4529]. In fibrosis, TGF-β1 induces Smad 2/3 phosphorylation by binding to their membrane receptors, followed by binding with Smad 4 to form a complex that translocates to the nucleus. This signaling cascade finally produces fibrotic mediators such as α-SMA and collagens. In particular, CS promotes fibrotic response via the activation of TGF-β1/Smad signaling, which is observed in patients with COPD, especially in those who smoker [303132]. However, Cho et al. [11] reported that a mixture of antioxidants including PYC significantly reduced ultraviolet radiation-induced wrinkle formation by preventing the expression of matrix metallo-proteinases, TGF, and Type I procollagen. Collectively, this this evidence suggests that PYC has a potential to suppress pulmonary fibrosis induced by CS by the inhibition of TGF-β1/Smad 2/3 signaling.
In conclusion, PYC markedly reduced the production of fibrotic mediators in CS+LPS exposed mice. These properties were closely associated with the suppression of TGF-β1/Smad 2/3 signaling. Therefore, our study suggests that PYC may effectively suppress pulmonary fibrosis and is a potential candidate worth further investigation for development as a therapeutic agent.
Figures and Tables
 | Figure 1PYC treatment inhibited the inflammatory cell counts in the mouse BALF. Cells were isolated by centrifugation and stained with the Diff-Quik staining reagent. NC, normal control mice; CS+LPS, CS- and LPS-induced mice; ROF, CS- and LPS-induced mice treated with roflumilast (10 mg/kg); PYC-10, CS- and LPS-induced mice treated with PYC (10 mg/kg); PYC-20, CS- and LPS-induced mice treated with PYC (20 mg/kg). The values are expressed as the means±SD (n=7). ##Significantly different from NC, P<0.01; *,**Significantly different from CS+LPS, P<0.05, P<0.01, respectively. |
 | Figure 2PYC treatment inhibited proinflammatory cytokine levels in the mouse BALF. IL-1β, IL-6, and TNF-α levels were determined by ELISA. NC, normal control mice; CS+LPS, CS- and LPS-induced mice; ROF, CS- and LPS-induced mice treated with roflumilast (10 mg/kg); PYC-10, CS- and LPS-induced mice treated with PYC (10 mg/kg); PYC-20, CS- and LPS-induced mice treated with PYC (20 mg/kg). The values are expressed as the means±SD (n=7). ##Significantly different from NC, P<0.01; **Significantly different from CS+LPS, P<0.01. |
 | Figure 3PYC treatment inhibited airway inflammation. Histological examination of airway inflammation, performed in lung tissue using H&E staining. NC, normal control mice; CS+LPS, CS- and LPS-induced mice; ROF, CS- and LPS-induced mice treated with roflumilast (10 mg/kg); PYC-10, CS- and LPS-induced mice treated with PYC (10 mg/kg); PYC-20, CS- and LPS-induced mice treated with PYC (20 mg/kg). The values are expressed as the means±SD (n=7). ##Significantly different from NC, P<0.01; *,**Significantly different from CS+LPS, P<0.05, P<0.01, respectively. |
 | Figure 4PYC treatment inhibited collagen deposition. Histological examination of collagen deposition (blue color), performed in lung tissue using Masson's trichrome staining. NC, normal control mice; CS+LPS, CS- and LPS-induced mice; ROF, CS- and LPS-induced mice treated with roflumilast (10 mg/kg); PYC-10, CS- and LPS-induced mice treated with PYC (10 mg/kg); PYC-20, CS- and LPS-induced mice treated with PYC (20 mg/kg). The values are expressed as the means±SD (n=7). ##Significantly different from NC, P< 0.01; *,**Significantly different from CS+LPS, P<0.05, P< 0.01, respectively. |
 | Figure 5PYC treatment inhibited TGF-β1 and collagen expression levels in lung tissue of mice. (A) Immunohistochemical analysis for TGF-β1 positivity performed in lung tissue. (B) Immunohistochemical analysis for collagen positivity performed in lung tissue. NC, normal control mice; CS+LPS: CS- and LPS-induced mice; ROF, CS and LPS-induced mice treated with roflumilast (10 mg/kg); PYC-10, CS- and LPS-induced mice treated with PYC (10 mg/kg); PYC-20, CS- and LPS-induced mice treated with PYC (20 mg/kg). The values are expressed as the means±SD (n=7). ##Significantly different from NC, P<0.01; **Significantly different from CS+LPS, P<0.01. |
 | Figure 6PYC reduced TGF-β1 and collagen protein levels and Smad 2/3 phosphorylation in lung tissue. Protein expression was assessed by western blot analysis and relative ratio of protein expression was evaluated. β-Actin was used as a protein loading control. NC, normal control mice; CS+LPS, CS- and LPS-induced mice; ROF, CS- and LPS-induced mice treated with roflumilast (10 mg/kg); PYC-10, CS- and LPS-induced mice treated with PYC (10 mg/kg); PYC-20, CS- and LPS-induced mice treated with PYC (20 mg/kg). The values are expressed as the means±SD (n=7). ##Significantly different from NC, P<0.01; *,**Significantly different from CS+LPS, P<0.05, P<0.01, respectively. |
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (grant number: NRF-2016R1C1B2008818).
References
1. Churg A, Tai H, Coulthard T, Wang R, Wright JL. Cigarette smoke drives small airway remodeling by induction of growth factors in the airway wall. Am J Respir Crit Care Med. 2006; 174(12):1327–1334.
2. Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001; 164:S28–S38.
3. Milara J, Peiró T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013; 68(5):410–420.
4. Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med. 2003; 31(4):S258–S264.
5. Xu F, Liu C, Zhou D, Zhang L. TGF-â/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016; 64(3):157–167.
6. Brandsma CA, Timens W, Jonker MR, Rutgers B, Noordhoek JA, Postma DS. Differential effects of fluticasone on extracellular matrix production by airway and parenchymal fibroblasts in severe COPD. Am J Physiol Lung Cell Mol Physiol. 2013; 305(8):L582–L589.
7. Farid M, Kanaji N, Nakanishi M, Gunji Y, Michalski J, Iwasawa S, Ikari J, Wang X, Basma H, Nelson AJ, Liu X, Rennard SI. Smad3 mediates cigarette smoke extract (CSE) induction of VEGF release by human fetal lung fibroblasts. Toxicol Lett. 2013; 220(2):126–134.
8. Ko JW, Lee IC, Park SH, Moon C, Kang SS, Kim SH, Kim JC. Protective effects of pine bark extract against cisplatin-induced hepatotoxicity and oxidative stress in rats. Lab Anim Res. 2014; 30(4):174–180.
9. Matsumori A, Higuchi H, Shimada M. French maritime pine bark extract inhibits viral replication and prevents development of viral myocarditis. J Card Fail. 2007; 13(9):785–791.
10. Mei L, Mochizuki M, Hasegawa N. Hepatoprotective effects of pycnogenol in a rat model of non-alcoholic steatohepatitis. Phytother Res. 2012; 26(10):1572–1574.
11. Cho HS, Lee MH, Lee JW, No KO, Park SK, Lee HS, Kang S, Cho WG, Park HJ, Oh KW, Hong JT. Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation. Photodermatol Photoimmunol Photomed. 2007; 23(5):155–162.
12. Shin NR, Ryu HW, Ko JW, Park JW, Kwon OK, Oh SR, Kim JC, Shin IS, Ahn KS. A standardized bark extract of Pinus pinaster Aiton (Pycnogenol®) attenuated chronic obstructive pulmonary disease via Erk-sp1 signaling pathway. J Ethnopharmacol. 2016; 194:412–420.
13. Tashkin DP. Roflumilast: the new orally active, selective phophodiesterase-4 inhibitor, for the treatment of COPD. Expert Opin Pharmacother. 2014; 15(1):85–96.
14. Shin IS, Shin NR, Park JW, Jeon CM, Hong JM, Kwon OK, Kim JS, Lee IC, Kim JC, Oh SR, Ahn KS. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J Pineal Res. 2015; 58(1):50–60.
15. Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2001; 163(6):1476–1483.
16. Eltom S, Belvisi MG, Stevenson CS, Maher SA, Dubuis E, Fitzgerald KA, Birrell MA. Role of the inflammasome-caspase1/11-IL-1/18 axis in cigarette smoke driven airway inflammation: an insight into the pathogenesis of COPD. PLoS One. 2014; 9(11):e112829.
17. Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004; 1(1):59–70.
18. Ryu HW, Song HH, Shin IS, Cho BO, Jeong SH, Kim DY, Ahn KS, Oh SR. Suffruticosol A isolated from paeonia lactiflora seedcases attecuates airway inflammation in mice induced by cigarette smoke and LPS exposure. J Funct Foods. 2015; 17:774–784.
19. Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Tissue renewal and repair: regeneration, healing, and fibrosis. Pathologic basis of disease. 2005; 7:87–118.
20. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008; 214(2):199–210.
21. Peng YJ, Lee CH, Wang CC, Salter DM, Lee HS. Pycnogenol attenuates the inflammatory and nitrosative stress on joint inflammation induced by urate crystals. Free Radic Biol Med. 2012; 52(4):765–774.
22. Khan MM, Kempuraj D, Thangavel R, Zaheer A. Protection of MPTP-induced neuroinflammation and neurodegeneration by Pycnogenol. Neurochem Int. 2013; 62(4):379–388.
23. Shin IS, Shin NR, Jeon CM, Hong JM, Kwon OK, Kim JC, Oh SR, Hahn KW, Ahn KS. Inhibitory effects of Pycnogenol® (French maritime pine bark extract) on airway inflammation in ovalbumin-induced allergic asthma. Food Chem Toxicol. 2013; 62:681–686.
24. Xia YF, Zhang JH, Xu ZF, Deng XM. Pycnogenol, a compound isolated from the bark of pinus maritime mill, attenuates ventilator-induced lung injury through inhibiting NF-κB-mediated inflammatory response. Int J Clin Exp Med. 2015; 8(2):1824–1833.
25. Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. Am J Physiol Cell Physiol. 2014; 306(11):C987–C996.
26. Gao W, Li L, Wang Y, Zhang S, Adcock IM, Barnes PJ, Huang M, Yao X. Bronchial epithelial cells: The key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology. 2015; 20(5):722–729.
27. Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med. 2005; 26(2):142–153.
28. Zhang H, Liu H, Borok Z, Davies KJ, Ursini F, Forman HJ. Cigarette smoke extract stimulates epithelial-mesenchymal transition through Src activation. Free Radic Biol Med. 2012; 52(8):1437–1442.
29. Vietti G, Lison D, van den Brule S. Mechanisms of lung fibrosis induced by carbon nanotubes: towards an Adverse Outcome Pathway (AOP). Part Fibre Toxicol. 2016; 13(1):11.
30. Zandvoort A, Postma DS, Jonker MR, Noordhoek JA, Vos JT, van der Geld YM, Timens W. Altered expression of the Smad signalling pathway: implications for COPD pathogenesis. Eur Respir J. 2006; 28(3):533–541.
31. Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration. 2011; 81(4):265–284.
32. Mahmood MQ, Reid D, Ward C, Muller HK, Knight DA, Sohal SS, Walters EH. Transforming growth factor (TGF) β1 and Smad signalling pathways: A likely key to EMT-associated COPD pathogenesis. Respirology. 2017; 22(1):133–140.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download