This article has been
cited by other articles in ScienceCentral.
Abstract
The genetically engineered mice require special husbandry care and are mainly housed in Individually Ventilated Cage (IVC) systems and Static Micro Isolator Cages (SMIC) to minimize the risk for spreading undesirable microorganisms. However, the static micro isolation cage housing like SMIC are being replaced with IVC systems in many facilities due to a number of benefits like a higher density housing in limited space, better protection from biohazards and allergens and decreased work load due to decreased frequency of cage changing required in this system. The purpose of this study was to examine the reproductive performance of genetically engineered mice housed in individually ventilated cages (IVC) and Static Micro Isolator Cages (SMIC). When the B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax transgenic mice were housed in these two housing systems, the number of litters per dam, number of pups born per dam and number of pups weaned per dam were found to be slightly higher in the IVC as compared to the SMIC but the difference was not significant (P<0.05). In case of Growth Associated Protein 43 (GAP-43) knockout mice, the number of litters born per dam and the number of pups born per dam were marginally higher in the IVC as compared to those housed in SMIC but the difference was not significant (P<0.05). Only the number of pups weaned per dam were found to be significantly higher as compared to those housed in the SMIC system at P<0.05.
Go to :

Keywords: Reproductive Performance, Genetically Engineered Mice, Housing System
Genetic engineering is the process of manipulation of genetic composition of organisms after insertion or deletion of genes of desired traits. It is also widely used field to study the function of these set of genes being manipulated. This technique is of great importance in many aspects of biomedical science including gene regulation, cancer research, immune system, developmental biology, biomedicine and agriculture. Genetically engineered mice may require special care and housing at times depending on the mutation being introduced and change in the phenotype of model when compared to normal mice and are mainly housed and bred in Individually Ventilated Cages (IVC) and Static Micro Isolator Cages (SMIC) to minimize the risk for spread of undesirable microorganisms.
Potential benefits of IVC systems include protection of small groups of animals against infections, protection of the environment from the animals and compensation for poor air change rates in the room [
1]. IVC systems help to maintain low ammonia and CO
2 concentrations, support a low relative humidity and reduce spread of infective agents and allergenic contaminants. The cage environment is the key factor for reproduction in laboratory animals as breeding may be affected by levels of ammonia, CO
2, relative humidity, airflow and cross contamination. In IVC systems each cage receives HEPA filtered air supply at a ventilation rate that may be adjusted from around 25 to 120 air changes per hour, which helps in maintaining the integrity of the cage microenvironment and also helps in reducing environmental contamination and pollutants with the help of HEPA filtered exhaust air. However, these systems may also have welfare implications for the animals due to the high intracage ventilation [
2]. It has been also reported in a study conducted on C57BL6/J mice that a slight change in environment can lead to poor reproductive performance, due to stress and there is more risk of cannibalism of the pups [
3].
Static Micro Isolator Cages (SMIC) are effective in preventing microbial contamination of the animals housed in these cages [
4]. Consequently, these cages are widely used to house specific pathogen-free rodents. However, experimental studies have demonstrated that the filtertop interferes with intra-cage ventilation and microenvironmental levels of carbon dioxide and ammonia can reach detrimental levels [
56].
B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax is a double transgenic mice which express a chimeric mouse/human amyloid precursor protein (Mo/HuAPP695swe) and a mutant human presenilin 1(PS1-dE9) both directed to central nervous system (CNS) neurons. Both mutations are associated with early-onset of Alzheimer's disease. These transgenic mice develop beta-amyloid deposits in brain by six to seven months of age. Homozygous mice on the C57BL/6 background (N9B6) exhibit a high incidence of seizures which initiate at 3 to 3.5 months of age and increase further at around 4.5 months of age. 10-15% mortality is reported for transgenic mice on the congenic (N9) C57BL/6 background [
7]. Homozygous mice, on the congenic C57BL/6J background (N13) at 17-18 weeks in age, exhibit epileptiform discharges and mortality was 38%. It has been reported that the phenotypes produced by expression of human amyloid precursor protein (APP) transgenes vary depending on the genetic background of the mouse and may be different even within a strain depending on the sub strain. APP-induced premature death is quite high (50% or more depending on the location) with APP transgenes on B6, DBA/2J, FVB and other strains [
89]. This might be one of the reasons for the low pup survival in this particular transgenic mice strain. These B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice are useful in studies of neurological disorders of the brain, specifically Alzheimer's disease, amyloid plaque formation and aging. The breeding colony of these transgenic mice was established at Animal Facility of National Brain Research Centre (NBRC) and maintained by breeding homozygotes with wild type siblings.
Growth-associated protein (GAP-43) encoded by the GAP 43 gene in humans [
10] is reported to regulate the response of neurons to axonal guidance signals and also has a possible role in neurogenesis. Metz and Schwab have reported that GAP-43 plays a major role in the developmental organization of the central nervous system (CNS) in vivo and those mice which do not express GAP-43 show extensive motor and sensory impairment [
11]. Gap 43 knockout mice have been used as models to study the function of GAP43 protein. However, it has been reported that survival of the homozygous mutant mice is greatly decreased and only 10% of the animals without GAP-43 survive past 3 weeks [
12]. These authors also suggest that reproductive-related behavior is abnormal, although observations are necessarily limited because only a very few homozygous mice reach maturity. Shen et.al. showed that 100% of homozygote GAP-43 (-/-) mice failed to form the anterior commissure (AC), hippocampal commissure (HC), and corpus callosum (CC) in vivo [
13] and that the cerebellum is also affected [
6]. Several other studies have also reported that most homozygous GAP-43 knockouts die soon after birth and are thus precluded from behavioral studies [
141516]. Rekart et al proposed that the level of GAP-43 determines the achievement level of memory-associated performance in a bidirectional manner i.e. low GAP-43 levels impair and high levels, but not beyond an optimum point, enhance performance [
1617]. The GAP 43 Knockout mice breeding colony was established at Animal Facility of National Brain Research Centre and maintained by mating heterozygote males and females.
The purpose of this study was to examine the reproductive performance of these two genetically engineered mice i.e B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice and GAP-43 Knock Out mice housed in individually ventilated cages (IVC) and Static Micro Isolator Cages (SMIC).
Materials and Methods
Mice
220 breeding pairs each of B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice and Growth Associated Protein-43 Knock Out mice (GAP-43 KO) housed in the two different housing systems (110 pairs/housing system) were evaluated for their breeding performance in the breeding colonies maintained in the Animal Facility of National Brain Research Centre (NBRC), Manesar, Gurgaon, Haryana, India. The Breeding nucleus of B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax breeders were imported from The Jackson laboratories, 600 Main St, Bar Harbor, ME 04609, USA and breeding colonies established at NBRC for use of this model by neuroscientists of the institute.
The breeding nucleus of 10 breeders of GAP 43 Knockout mice were imported from Tufts University, Boston, MA, 02111 for establishing a colony at National Brain Research Centre , Manesar, India for scientists who were interested in studying the molecular mechanisms by which GAP 43 regulated cerebellar shape. The GAP 43 gene mutation was created on 129/Sv inbred and C57Bl6 backcross background at the Tufts University, Boston and has been referred to as GAP 43 Knockout mice.
Ethics Statement
The Animal Facility has approval and registration from the Committee for Purpose of Control and Supervision of Experiments on Animals (CPCSEA), the regulatory body in India. The breeding colonies of these two genetically engineered strains of mice were established in the facility after ethical approval from Institutional Animal Ethics Committee (IAEC) for the studies in which they had to be used by the investigators and animal husbandry and care was provided in accordance with the CPCSEA guidelines. Observations reported here were noted during the course of routine program of animal breeding and maintenance undertaken to meet the requirement of NBRC scientists.
Housing
The mice were housed in two different housing systems: Individually Ventilated Cage (IVC) system with cage Dimensions (W×D×H) 403 mm×165 mm×174 mm (Tecniplast, Italy) and Static Micro Isolator Cages (SMIC) with cage dimensions (267 mm×207 mm×140 mm) (Tecniplast, Italy). The mice were housed under barrier conditions in IVC or SMIC in the same room with all material coming in direct contact with the mice like feed, water, bedding, cages and nesting material being sterilized. The access to the room where these mice were housed was restricted only to the animal care staff and veterinarian who would wear all personal protective equipment like scrubs, masks, hair caps and sterile gloves to prevent spread of any infection to these mice. Both types of cages were changed only under a laminar flow and hands were disinfected before handling animals of each cage.
These mice colonies were housed at a room temperature of 22±3℃, relative humidity between 45-65% and light dark cycle of 12/12 hours. The IVC's were maintained at 60 air changes per hour for both the strains of mice and the location of air inlet and outlet for IVC cages were in the top of the cage cover so that there were no air drafts at animal level, avoiding risk of stress in them. The SMIC was maintained in positive pressure room with 10-12 air changes per hour for both the groups. The breeders were house at a maximum of 1 male and 2 females per cage and adults at a maximum of 4 adult males or females in stock in both the systems. The IVC were changed at a frequency of once a week whereas the SMIC was changed twice a week with fresh autoclaved cages. All the cages with pregnant females about to deliver were observed daily from a distance for presence of pups and date of delivery was mentioned on the cage cards. However, these cages remained unchanged with same microenvironment for at least 6 days after birth exception being dead or cannibalized pups were found in observation. All cage changes and animal handling was done in laminar flow (Atlantis, India).
Autoclaved paddy husk was used as bedding material in all cages. Paddy husk is the outer husk of Rice/Paddy seeds and is popularly used as bedding for laboratory animals in India because of its absorbent properties, it is autoclavable and has low cost compared to other bedding available in India. Commercial Pellet diet (Golden Feeds, Mehrauli, India) containing approximately 20% protein, 5% fat and 3% fibre, was irradiated and given ad libitum to all mice. Autoclaved RO water was provided to all mice. Shredded paper was autoclaved and provided as nesting material to all breeders and the familiar nesting material was transferred to the clean cage while cage changing as part of the cleaning routine.
Experimental Design
Initially, mice were selected for breeding at the age of 6-8 week's age based on genotyping results and randomly distributed to the two housing systems i.e. IVC or SMIC. The males and females were housed together in the same housing system till the time they continued to breed. The breeders were retired only when pups were not delivered for more than two months after weaning of the previous litter.
The pups were weaned at 21 days of age, individually identified by ear tags and housed at a maximum of 4 adult male or female mice per cage in the same housing system in which they were born. New breeding pairs were again established at the age of 6-8 weeks after genotyping and housed in the same housing system in which they were born. In this way, reproductive performance of 220 breeding pairs of B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice and 220 breeding pairs of GAP-43 KO mice housed in the two different housing systems (110 pairs/strain/housing system) was evaluated. Recording was done for the total number of litters born, pups born and pups weaned in the 110 breeders of B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice and GAP-43 KO mice housed in the IVC and SMIC and average number of litters per dam, number of pups born per dam and number of pups weaned per dam were further calculated. The females that did not get pregnant were not included in the study. Both the housing systems were kept in the same room under barrier conditions wherein everything coming in direct contact with the mice was sterilized and were maintained by same animal care staff for side-to-side comparisons.
Statistical Analysis
A GLM model (Binomial logistic regression) was built to decide if different cage setups are significantly associated with measured variables (Number of litters born per dam, number of pups born per dam and number of pups weaned per dam. Models were built separately for B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice and GAP 43 KO mice for testing the significance of measured variables with the cage setup. Models were implemented in R programming software using glm functions.
Go to :

Results
The reproductive performance of B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice and GAP-43 KO mice housed in IVC and SMIC was evaluated by recording the total number of litters born, pups born and pups weaned in the 110 breeders. An average number of litters per dam, number of pups born per dam, number of pups weaned per dam and pup survival till weaning were further calculated. Initially, mice were selected for breeding at the age of 6-8 weeks' age based on genotyping results and randomly distributed to the two housing systems i.e. IVC or SMIC. The males and females were housed together in the same housing system till the time they continued to breed. New breeding pairs were again established at the age of 6-8 weeks after genotyping and housed in the same housing system in which they were born. Further the breeders were retired only when pups were not delivered for more than two months after weaning of the previous litter.
Number of litters
The 110 B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax breeders housed in the IVC produced a total of 280 litters as compared to 271 litters produced by the same number of B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax females housed in SMIC. The average number of litters born per dam was 2.54 and 2.46 in the IVC and SMIC respectively, which was marginally more in the IVC as compared to SMIC but the difference, was not significant (
P value=0.276) at threshold p value of 0.05. (
Tables 1,
2,
Figure 1)
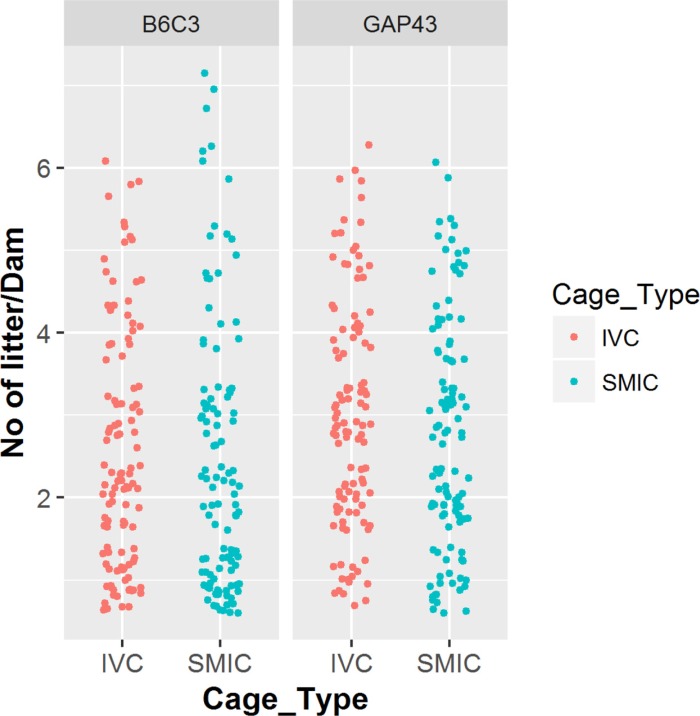 | Figure 1No. of litter per dam: Figure represents the no of litter per dam for mice strains B6C3-Tg (APPswe,PSEN1dE9) 85Dbo/Mmjax (Left Panel) and GAP 43 KO mice (Right Panel) under two different cage types IVC and SMIC.
|
Table 1
Total and average number of litters born per dam, pups born per dam, pups weaned per dam and Pup survival till weaning (%) in B6C3-Tg (APPswe,PSEN1dE9) 85Dbo/Mmjax mice housed in IVC and SMIC cages

|
B6C3 - Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice |
|
IVC |
SMIC |
|
Total number of litters born in 110 breeders |
280 |
271 |
|
Average number of litters per dam |
2.54 |
2.46 |
|
Total number of pups born in 110 breeders |
1690 |
1568 |
|
Average number of pups born per dam |
15.36 |
14.25 |
|
Total number of pups weaned in 110 breeders |
703 |
599 |
|
Average number of pups weaned/dam |
6.39 |
5.44 |
|
Pup survival till weaning (%) |
41.6 |
38.2 |

Table 2
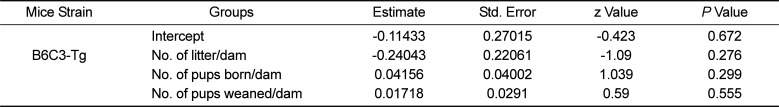
Statistical analysis of data for B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice housed in SMIC and IVC. In case of B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice none of the variables were found to be significantly associated with the type of housing (SMIC and IVC) at threshold P value of 0.05
|
Mice Strain |
Groups |
Estimate |
Std. Error |
z Value |
P Value |
|
B6C3-Tg |
Intercept |
-0.11433 |
0.27015 |
-0.423 |
0.672 |
|
No. of litter/dam |
-0.24043 |
0.22061 |
-1.09 |
0.276 |
|
No. of pups born/dam |
0.04156 |
0.04002 |
1.039 |
0.299 |
|
No. of pups weaned/dam |
0.01718 |
0.0291 |
0.59 |
0.555 |

In case of GAP-43 KO mice, the total number of litters produced by the 110 females housed in IVC was 331 whereas the same number of females housed in SMIC produced 305 litters. The average number of litters born per dam was 3.01 in the IVC, which was slightly more than that in SMIC where it was 2.77 litters born per dam but the difference was not significant (
P value=0.75). (
Tables 3,
4,
Figure 1).
Table 3
Total and average number of litters born per dam, number of pups born per dam, number of pups weaned/per dam and Pup survival till weaning (%) in GAP 43 knockout mice housed in IVC and SMICcages.

|
Groups |
GAP-43 KO
Mice |
|
IVC |
SMIC |
|
Total number of litters born in 110 breeders |
331 |
305 |
|
Average number of litters per dam |
3.01 |
2.77 |
|
Total number of pups born from 110 breeders |
2127 |
1882 |
|
Average number of pups born per dam |
19.34 |
17.11 |
|
Total number of pups weaned from 110 breeders |
1156 |
846 |
|
Average number of pups weaned from 110 breeders |
10.51 |
7.69*
|
|
Pup survival till weaning (%) |
54.35 |
44.9 |

Table 4
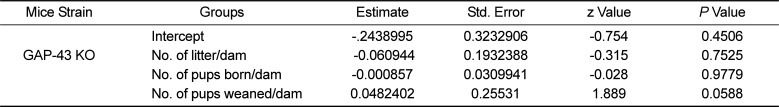
Statistical analysis of data GAP 43 KO mice housed in SMIC and IVC. On statistical analysis in case of GAP43 KO mice, only the number of pups weaned/dam was found to be significantly higher in those housed in IVC as compared to those housed in SMIC at alpha 0.1. (P value=0.058)
|
Mice Strain |
Groups |
Estimate |
Std. Error |
z Value |
P Value |
|
GAP-43 KO |
Intercept |
-0.2438995 |
0.3232906 |
-0.754 |
0.4506 |
|
No. of litter/dam |
-0.060944 |
0.1932388 |
-0.315 |
0.7525 |
|
No. of pups born/dam |
-0.000857 |
0.0309941 |
-0.028 |
0.9779 |
|
No. of pups weaned/dam |
0.0482402 |
0.25531 |
1.889 |
0.0588 |

Number of pups born
The 110 B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax females which were housed in the IVC produced a total of 1690 pups during their entire breeding life as compared to 1568 pups produced by the same number of breeders housed in the SMIC. The average number of pups born per dam in the IVC was 15.36 as compared to 14.25 in SMIC but the difference was not found to be significant (
P=0.299). (
Tables 1,
2,
Figure 2)
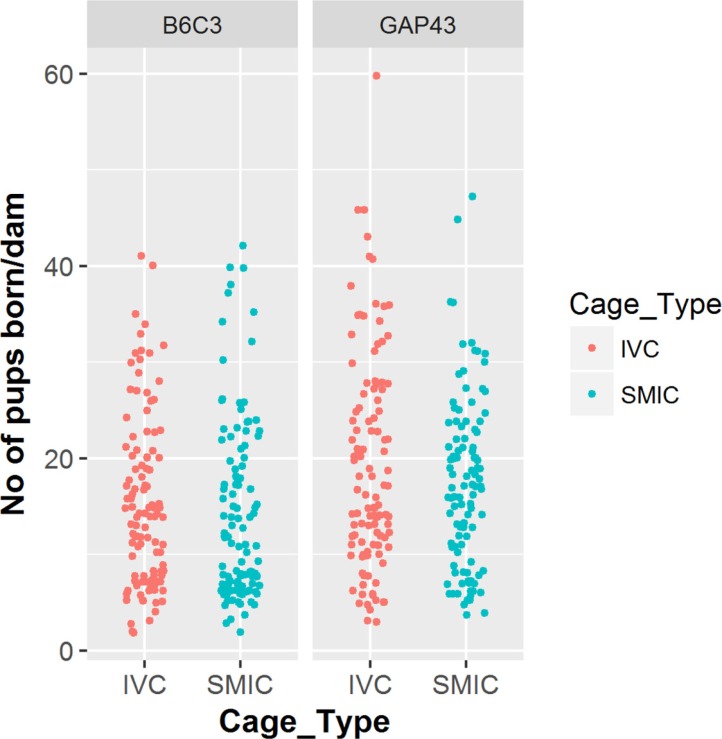 | Figure 2No. of pups born per dam: Figure represents the no of pups born per dam for mice strains B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax (Left Panel) and GAP 43 KO mice (Right Panel) under two different cage types IVC and SMIC.
|
In case of 110 GAP-43 KO breeders housed in IVC cages, a total of 2127 pups were born and same number of breeders housed in SMIC gave birth to a total of 1882 pups. The average number of pups born per dam in IVC was 19.34 as compared to 17.11 in SMIC but the difference was not found to be significant (
P value=0.977) at a threshold p value of 0.05 (
Tables 3,
4,
Figure 2).
Number of pups weaned
The number of pups which survived up to weaning age was 703 in case of 110 B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax females housed in IVC as compared to 599 pups weaned from females housed in the SMIC. The average number of pups weaned from IVC and SMIC cages was 6.39 and 5.44 respectively. These values were not found to be significantly different (
P value=0.555) (
Tables 1,
2,
Figure 3).
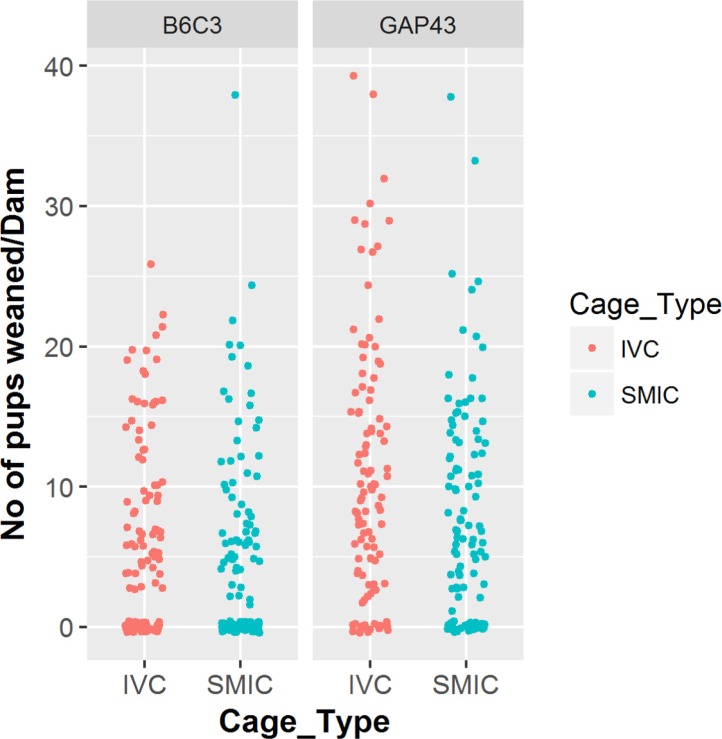 | Figure 3No. of pups weaned per dam: Figure represents the no of pups weaned per dam for mice strains B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax (Left Panel) and GAP 43 KO mice (Right Panel) under two different cage types IVC and SMIC.
|
In GAP-43 KO colony a total of 1156 pups were weaned from the 110 breeders housed in IVC and 846 pups were weaned from the breeders housed in SMIC cages. The average number of pups weaned from females housed in IVC was 10.51 which was significantly higher at alpha level 0.1 (
P value=0.058) as compared to 7.69 pups weaned per dam in SMIC (
Tables 3,
4,
Figure 3).
Pup survival till weaning (%)
The rate of pup survival till weaning was calculated by comparing the number of pups survived until weaned to the number of pups born as shown below:
The number of pups survived until weaned from the 110 B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice breeders was 703 out of the total of 1690 pups which were born leading to a pup survival till weaning (%) of 41.60%. Amongst110 breeders housed in SMIC, 599 pups survived till weaning from 1568 pups born leading to a pup survival percentage of 38.20% which was not found to be significantly less as compared to that in mice housed in IVC (
Tables 1,
2,
Figure 4).
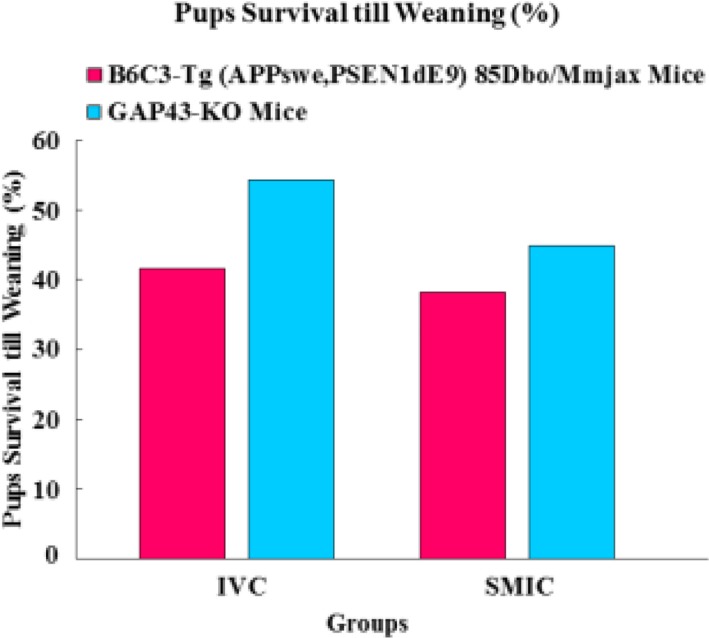 | Figure 4Pup Survival till Weaning (%) in B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax and GAP 43 KO mice housed in SMIC and IVC. (Calculated as Total Number of Pups Survived till Weaned/Total Number of Pups Born ×100)
|
In case of GAP 43 KO mice, 1156 pups survived till weaning from a total of 2127 pups born from the 110 breeders housed in IVC resulting in a pup survival rate of 54.35% which was significantly higher (
P=0.0588). In the 110 breeders housed in SMIC, 846 pups survived till weaning out of the total 1882 pups born leading to a survival rate ate of 44.9% (
Tables 3,
4,
Figure 4).
Go to :

Discussion
The conventional open housing and static micro isolation cage housing is being replaced with IVC systems in many facilities and the environmental conditions in these two housing systems have been compared by several researchers. The IVC have a number of benefits like a higher degree of containment, better protection from allergens and decreased work load due to decreased frequency of cage changing required in this system. However, the microenvironment in IVC cages is dependent on factors like ventilation rates (air changes per hour), air distribution, number of mice housed per cage and frequency of bedding change. The main objective of this study was to evaluate the reproductive performance of genetically engineered mice housed in IVC and SMIC.
When the B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice were housed in these two housing systems with a cage change frequency of once a week in IVC and twice a week in SMIC, the number of litters per dam, number of pups born per dam and number of pups weaned per dam were found to be only marginally higher in the IVC as compared to the SMIC but the difference was not significant. Similarly, in case of the GAP-43 KO mice also the number of litter per dam and number of pups born per dam were only marginally higher in the IVC as compared to those housed in SMIC and the difference was not significant. However, the number of pups weaned per dam was found to be significantly higher in IVC as compared to those housed in the SMIC system.
SMIC cause accumulation of waste gases like carbon dioxide and ammonia as well as increased intracage relative humidity [
6]. Previous studies have reported that intracage air temperatures also increase due to the number and size of the mice in the cage, use of filter tops on static caging, and air circulation within the cage [
361819]. However an increased frequency of cage change in the SMIC adopted in this study might have been helpful in avoiding accumulation of waste gases and prevented increase in the intracage relative humidity and temperature. Further, the reproductive performance of these transgenic mice in IVC may be improved further by reducing the air changes per hour (ACH). Reeb et al. (1998) reported that ventilation rates of 30 ACH were sufficient to maintain an adequate microenvironment when bedding was changed weekly while increasing the ventilation rates to 60 ACH was required if cage change frequency was reduced to every two weeks. As in the present study the IVC cages were being changed once a week reducing the ventilation rates from 60 ACH might be helpful in increasing the breeding performance as preference testing has also shown that when given the opportunity, mice have avoided cages with higher ventilation rates [
2].
It was found that the pup survival percentage in the B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice housed in IVC was only marginally more as compared to those housed in SMIC though the overall survival percentages for this transgenic strain were less in both the housing systems, ranging from 41.60% in IVC to 38.20% in SMIC. In GAP 43 KO colony, pup survival percentage in breeders housed in IVC was significantly more when compared to those housed in SMIC. However, the overall pup survival percentage for these GAP 43 knockout mice was less in both the housing systems ranging from 54.35% in IVC to 44.9% in SMIC.
It is therefore suggested that researchers who are planning to upgrade their mice breeding facilities from SMIC to IVC systems for increased breeding efficiency might consider the potential impact that this new housing system may have on the specific genetically engineered mice models as well as the costs involved in this up gradation of the housing system.
Go to :

Conclusion
In this study the reproductive performance of the genetically engineered mice housed in IVC was compared side to side with those housed in the SMIC. The breeding performance of the 110 B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/Mmjax mice breeders housed in IVC in terms of the number of litters per dam, number of pups born per dam and number of pups weaned per dam was found to be marginally more as compared to the 110 breeders housed in the SMIC but the difference was not significant. In case of the 110 GAP-43 KO mice breeders housed in IVC also the number of litters born per dam and number of pups born per dam were marginally more as compared to SMIC. But the difference was not significant and only the number of pups weaned per dam was found to be significantly higher in the IVC system as compared to the SMIC system.
Based on this study, it may be concluded that the animal facilities who cannot afford the high cost of an IVC system may use SMIC as an economic alternative for housing and breeding genetically engineered mice if they change these cages twice a week, which might be helpful to avoid accumulation of high ammonia and carbon dioxide levels. Also further study made be undertaken to see if the breeding performance of these genetically engineered mice housed in the IVC system may be further improved by reducing the Air Changes per Hour (ACH) from 60 to 30 when the cages are changed once a week. Further, similar studies may also be performed on a number of other genetically engineered mice to compare the effect of the different housing systems on their breeding efficiency.
Go to :

Acknowledgment
Authors acknowledge Neeraj Jain, Scientist, National Brain Research Centre, Manesar, Haryana, India for his critical viewpoints about the literature and content for the article.
Go to :

Notes
Go to :

References
1. Brandstetter H1, Scheer M, Heinekamp C, Gippner-Steppert C, Loge O, Ruprecht L, Thull B, Wagner R, Wilhelm P, Scheuber HP. Working Group on Evaluation of IVC Systems of the Animal Welfare Information Center for Biomedical Research of the Faculty of Medicine. Performance evaluation of IVC systems. Lab Anim. 2005; 39(1):40–44. PMID:
15703123.

2. Baumans V, Schlingmann F, Vonck M, van Lith HA. Individually ventilated cages: beneficial for mice and men? Contemp Top Lab Anim Sci. 2002; 41(1):13–19.
3. Reeb C, Jones R, Bearg D, Bedigan H, Myers D, Paigen B. Microenvironment in Ventilated Animal Cages with Differing Ventilation Rates, Mice Populations, and Frequency of Bedding Changes. Contemp Top Lab Anim Sci. 1998; 37(2):43–49.
4. Sedlacek R, Orcutt R, Suit H, Rose E. Rose. Tokyo: Tokai University Press;1981. vol. 1:p. 151.
5. Keller LS, White WJ, Snider MT, Lang CM. An evaluation of intra-cage ventilation in three animal caging systems. Lab Anim Sci. 1989; 39(3):237–242. PMID:
2724925.
6. Lipman NS, Corning BF, Coiro MA Sr. The effects of intracage ventilation on microenvironmental conditions in filter-top cages. Lab Anim. 1992; 26(3):206–210. PMID:
1501435.
7. Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fülöp L, Penke B, Zilberter Y, Harkany T, Pitkänen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009; 29(11):3453–3462. PMID:
19295151.
8. Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK. Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet. 1997; 6(11):1951–1959. PMID:
9302276.

9. Krezowski J, Knudson D, Ebeling C, Pitstick R, Giri RK, Schenk D, Westaway D, Younkin L, Younkin SG, Ashe KH, Carlson GA. Identification of loci determining susceptibility to the lethal effects of amyloid precursor protein transgene overexpression. Hum Mol Genet. 2004; 13(18):1989–1997. PMID:
15254013.

10. Kosik KS, Orecchio LD, Bruns GA, Benowitz LI, MacDonald GP, Cox DR, Neve RL. Human GAP-43: its deduced amino acid sequence and chromosomal localization in mouse and human. Neuron. 1988; 1(2):127–132. PMID:
3272162.
11. Metz GA, Schwab ME. Behavioral characterization in a comprehensive mouse test battery reveals motor and sensory impairments in growth-associated protein-43 null mutant mice. Neuroscience. 2004; 129(3):563–574. PMID:
15541878.

12. Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995; 80(3):445–452. PMID:
7859286.

13. Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci. 2002; 22(1):239–247. PMID:
11756507.

14. Maier DL, Mani S, Donovan SL, Soppet D, Tessarollo L, McCasland JS, Meiri KF. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci USA. 1999; 96(16):9397–9402. PMID:
10430954.

15. Sretavan DW, Kruger K. Randomized retinal ganglion cell axon routing at the optic chiasm of GAP-43-deficient mice: association with midline recrossing and lack of normal ipsilateral axon turning. J Neurosci. 1998; 18(24):10502–10513. PMID:
9852588.

16. Rekart JL, Quinn B, Mesulam MM, Routtenberg A. Subfieldspecific increase in brain growth protein in postmortem hippocampus of Alzheimer's patients. Neuroscience. 2004; 126(3):579–584. PMID:
15183507.

17. Rekart JL, Meiri K, Routtenberg A. Hippocampal-dependent memory is impaired in heterozygous GAP-43 knockout mice. Hippocampus. 2005; 15(1):1–7. PMID:
15390153.

18. Laber K, Veatch LM, Lopez MF, Mulligan JK, Lathers DM. Effects of housing density on weight gain, immune function, behavior, and plasma corticosterone concentrations in BALB/c and C57BL/6 mice. J Am Assoc Lab Anim Sci. 2008; 47(2):16–23.
19. Lipman NS. Isolator Rodent Caging Systems (State of the Art): A Critical View. Contemp Top Lab Anim Sci. 1999; 38(5):9–17. PMID:
12086409.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download