Abstract
Quercetin, a natural flavonoid, copiously exists in vegetable, fruits and tea. Quercetin is beneficial to neurodegenerative disorders via its strong anti-oxidant and anti-inflammatory activities. γ-Enolase is one of the enzymes of glycolytic pathway and is predominantly expressed in neuronal cells. The aim of the present study is to verify whether quercetin modulates the expression of γ-enolase in brain ischemic injury. Adult Sprague-Dawley male rats were subjected to middle cerebral artery occlusion (MCAO) and quercetin (50 mg/kg) or vehicle was administered by intraperitoneal injection at 1 h before MCAO onset. A proteomics study, Western blot analysis, reversetranscription-PCR, and immunofluorescence staining were conducted to investigate the change of γ-enolase expression level. We identified a decline in γ-enolase expression in MCAO-operated animal model using a proteomic approach. However, quercetin treatment significantly attenuated this decline. These results were confirmed using Western blot analysis, reverse transcription-PCR, and immunofluorescence staining techniques. γ-Enolase is accepted as a neuron specific energy synthesis enzyme, and quercetin modulates γ-enolase in a MCAO animal model. Thus, our findings can suggest the possibility that quercetin regulates γ-enolase expression in response to cerebral ischemia, which likely contributes to the neuroprotective effect of quercetin.
Quercetin (3,5,7,30,40-pentahydroxyflavone) is a natural flavonoid that abundantly exists in vegetable, fruits and tea. It is reported that natural flavonoid is beneficial to many disorders such as neurodegenerative disorders, diabetes, and cancer via its strong anti-oxidant and anti-inflammatory activities [12]. It is well known that oxidative stress increases neuronal cell membrane breakdown [3]. Quercetin effectively protects the neuronal cells from the oxidative stress-induced neurodegeneration, decreases lipid peroxidation, prevents glutathione depletion, and improves the activity of catalase and superoxide dismutase [456]. Quercetin conserves neurons against oxidative stress and excitotoxicity by the modulation of cell death mechanisms [7]. Moreover, quercetin reduces apoptotic cell death in brain tissue of focal cerebral ischemia through the activation of brain derived neurotrophic factor and phosphoinositide 3 kinase (PI3K)/Akt signaling pathway [8].
Enolases are categorized as a glycolytic enzyme that participates in various cellular activities such as growth and differentiation [9]. Several functions are designated to the different isoforms of enolases according to their cellular localization. α-Enolase form occurs ubiquitously in most cells including macrophages and glial cells [1011]. β-Enolase exists in non-neuronal cells and is expressed exclusively in muscle cells [12]. γ-Enolase presents with its abundance in mature neurons and neuroendocrine cells, and so called neuronal specific enolase [13]. Also, γ-enolase is present in dendrites and amine precursor uptake and decarboxylation cells [14]. γ-Enolase is released to extracellular space from cells in destruction of neuronal cell membrane by injurious factor [151617]. Moreover, it is accepted as a biomarker related with brain diseases such as traumatic brain injury, stroke, hypoxic encephalopathy and epileptic seizure [15161819]. Therefore, we designed the experiments based on the hypothesis that ischemic damage would affect the expression level of γ-enolase and quercetin might exert its protective effect by the alteration of ischemia-induced changes in γ-enolase.
Adult Sprague-Dawley male rats (210–230 g, n=64) were purchased from Samtako Co (Animal Breeding Center, Osan, Korea). All experimental rats were given space with a controlled consistent temperature (25℃) and lighting environment (12 h/12 h light/dark cycle). All experimental protocols related to the use of animals were approved by the Institutional Animal Care and Use Committee at Gyeongsang National University. Animals were randomly divided into four groups as follows: vehicle+sham, quercetin+sham, vehicle+middle cerebral artery occlusion (MCAO), and quercetin+MCAO (n=13 per group). Quercetin (Sigma, St. Louis, MO, USA) was dissolved in phosphate-buffered saline containing 0.1% dimethyl sulfoxide. Quercetin (50 mg/kg) or vehicle was administered by intraperitoneal injection at 1 h before MCAO onse [20212223]. Vehicle only used a solvent solution without the quercetin addition.
Animals were anaesthetized with intramuscular injection of Zoletil (50 mg/kg, Virbac, Carros, France). Animals were placed in supine position on operating table and heat pad was placed to maintain the body temperature (37±0.5℃) during the operation. Ventral midline of the neck skin was incised to expose the right common carotid artery (CCA) and the external carotid artery (ECA). After a careful dissociation of right CCA and right ECA from the surrounding tissues, right CCA was temporarily blocked by microvascular clipping and the right ECA was cut. A flame-rounded 4/0 monofilament was carefully inserted into the cut of the right ECA. Nylon filament was advanced 20–22 mm from the right CCA bifurcation through the right internal carotid artery to occlude the right middle cerebral artery. Microvascular clip on the right CCA was removed carefully. After 24 h of MCAO onset, the animals were euthanized and decapitated in order to extract brain tissues.
The ischemic core of right cerebral cortex was homogenized on ice for 1 min with lysis buffer (8M urea, 4% CHAPS, ampholytes and 40 mM Tris-HCl). The proteins and cellular debris were separated by centrifugation at 16,000 g for 20 min at 4℃. The supernatants were collected and the extracted protein concentration was determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). Bovine serum albumin was used as a standard according to the manufacturer's instruction. Protein analysis was performed by 2-demensional gel electrophoresis. First-dimensional electrophoresis was performed through isoelectric focusing (IEF). Immobilized pH gradient (IPG) gel strips (17 cm, pH 4–7 and pH 6–9, Bio-Rad) containing 50 µg of protein sample were rehydrated for 13 h at room temperature using a sample buffer (8 M urea, 2% CHAPS, 20 mM DTT, 0.5% IPG buffer and bromophenol blue). Rehydrated strips were subjected to IEF using the Ettan IPGphor 3 System (GE Healthcare, Uppsala, Sweden) under the following conditions: 250 V for 15 min, 10,000 V for 3 h and then 10,000 to 50,000 V. Subsequently, strips were equilibrated via two steps. First step was performed in equilibration buffer [6 M urea, 30% glycerol, 2% SDS, 50 mM Tris-HCl (pH 8.8)] containing 1% DTT for 15 min and second step was processed in equilibration buffer containing 2.5% iodoacetamide for 15 min. After equilibration, strips were loaded onto SDS-polyacrylamide gradient gels (7.5–17.5%) for second dimensional electrophoresis. Gels were electrophoresed using Protein-II XI electrophoresis equipment until bromophenol blue dye reached the bottom under the following conditions: 5 mA for 2 h, followed by 10 mA for 10 h at 10℃. After electrophoresis, the gels were fixed in the fixation solution (12% acetic acid and 50% methanol) for 2 h and then immersed in 50% ethanol for 20 min. In order to visualize the protein spots, fixed gels were stained with a silver solution (0.2% silver nitrate) for 20 min and developed with a developer (0.2% sodium carbonate). Prior to analyzing the protein spots, gels were scanned by Agfa ARCUS 1200™ and stored as image forms (Agfa-Gevaert, Mortsel, Belgium). The gel images were analyzed with PDQuest 2-D analysis software (Bio-Rad). Targeted protein spots were extracted from gels to perform a MALDI-TOF. Trypsin-containing buffer was used for a gel digestion and proteins were extracted. Mass spectrometry was conducted on a Voyager System DE-STR MALDI-TOF mass spectrometer to analyze the extracted proteins. MS-Fit and ProFound programs were used to detect mass-analyzed proteins and SWISS-prot and NCBI databases were engaged to identify protein sequences.
Frozen ischemic core of right cerebral cortex was homogenized and sonicated on ice with lysis buffer [1% Triton X-100 and 1 mM EDTA in phosphate buffer saline (PBS, pH 7.4)] containing 200 µM phenylmethylsulfonyl fluoride. Subsequently, homogenates were centrifuged at 15,000 g for 20 min at 4℃ in order to separate the soluble proteins from lysates and supernatant was collected. The protein concentration of the supernatant was measured by BCA assay kit (Pierce, Rockford, IL, USA) with bovine serum albumin as a standard. Total protein samples (30 µg per lane) were loaded on 10% SDS-polyacrylamide gel and electrophoresed. Separated proteins were entirely transferred to poly-vinylidene fluoride membranes (Millipore, Billerica, MA, USA). In order to prevent the non-specific antibody reaction, membranes were blocked with 5% non-fat dried milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h. After blocking, membranes were washed with TBST and then incubated with primary antibodies: anti-γ-enolase (diluted 1:1000, Santa Cruz Biotechnology, TX, USA) and anti-β-actin (1:1,000, Santa Cruz Biotechnology). Membranes were washed with TBST three times to remove non-reactive primary antibodies. Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000, Pierce) were treated to the membranes as secondary antibodies. Immunoreactivity was identified by applying an enhanced chemiluminescence (ECL) Western blot analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, USA) according to the manufacturer's instruction. Western blot signal intensity was determined with SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, USA).
The Ischemic core of right cerebral cortex was homogenized with Trizol Reagent (Life Technologies, Rockville, MD, USA) and then centrifugation was performed at 13,000 g for 20 min at 4℃. Total RNA was collected by isolating the supernatant of the homogenates. Total RNA samples were transcribed into single-stranded complementary DNA using the Superscript III firststrand system (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendation. To amplify the targeted gene sequences of the cDNA, PCR was carried out under the following conditions: 5 min at 94℃; 30 sec at 94℃, 30 sec at 54℃, and 1 min at 72℃ for 30 cycles; and 10 min at 72℃. Primer sequences for γ-enolase and β-actin were 5′-TGGATCTCCATACTGCCAAAG-3′ (forward) and 5′-CCAACTCCTCTTCAATCCTCAT-3′ (reverse), and 5′-GGGTCAGAAGGACTCCTACG-3′ (forward) and 5′-GGTCTCAAACATGATCTGGG-3′ (reverse), respectively. PCR product was mixed with Loading STAR (Dyne bio, Sungnam, Korea) and loaded on 1% agarose gel for an electrophoresis. After electrophoresis, PCR product bands were visualized under the ultraviolet light. Intensity analysis of PCR product bands was carried out using SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, USA).
The brain samples were fixed in 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS, pH 7.4) solution. After dehydration and clearing processes with ethyl alcohol and xylene, tissues were embedded with Paraplast (Leica, Wetzlar, Germany) and sliced into 4 µm thickness using rotary microtome (Leica). The sliced sections were deparaffinized in xylene and hydrated with a series of differently concentrated ethyl alcohols (100, 95, 90, 80 and 70%) and water. Hydrated sections were submersed in 0.1M sodium citrate (pH 6.0) and autoclaved for antigen retrieval steps. After cooling the slides to room temperature, sections were treated with 0.5% fetal bovine serum for the blockade of non-specific bindings. The sections were incubated overnight at 4℃ with anti-γ-enolase (1:100, Santa Cruz Biotechnology). After the primary antibody incubation, slides were rinsed with PBS and fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:100, Santa Cruz Biotechnology) was reacted for 1 h at room temperature. Slides were mounted by using UltraCruz mounting medium with 4′,6-diamidino-2-phenylindole (DAPI, Santa Cruz Biotechnology) for DNA counterstaining and cover-slipped for microscopic evaluation. The fluorescent signal of slides was detected with a confocal microscope (FV-1000, Olympus, Tokyo, Japan) in a dark chamber and images were photographed for further data analysis. The five areas of ischemic core in right cerebral cortex were randomly selected in each animals and the number of γ-enolase positive cells was determined using Image-Pro Plus image analysis software. The ratio of γ-enolase positive cells was determined as the number of FITC-stained cells to the number of nuclei counterstained with DAPI.
We observed a change in γ-enolase protein spots in response to quercetin treatment during MCAO-induced cerebral ischemia using a proteomic approach. The peptide mass of γ-enolase was 14/70 and the sequence coverage was 34%. MCAO surgical injury induced the decrease of γ-enolase protein expression in the cerebral cortices. However, this decrease in γ-enolase expression by MCAO was attenuated in the presence of quercetin. γ-Enolase protein levels were similar between vehicle- and quercetin-treated animals that underwent the sham operation (Figure 1A). We evaluated γ-enolase levels as the ratio of the intensity of vehicle+sham to vehicle+MCAO animals and quercetin+MCAO animals, and the calculated values were 0.48±0.03 and 0.93±0.04, respectively (Figure 1B). Western blot and reverse transcription-PCR analyses showed changes in γ-enolase levels in response to quercetin during MCAO injury. γ-Enolase protein level was decreased in the MCAO-induced animals with vehicle treatment compared to the sham-operated group. Quercetin treatment alleviated the injury-induced decrease in γ-enolase protein expression (Figure 2A). The normalized γ-enolase protein levels by β-actin were 0.65±0.02 and 0.90±0.04 in vehicle+MCAO animals and quercetin+MCAO animals, respectively (Figure 2B). γ-Enolase transcription level was lower in the MCAO-induced animals with vehicle treatment than that of sham-operated animals, while quercetin treatment attenuated the injury-induced decrease in γ-enolase transcription level (Figure 3A). γ-Enolase transcription levels were similar in both vehicle- and quercetin-treated animals with sham operation. γ-Enolase transcription levels were 0.73±0.04 and 0.97±0.03 in vehicle+MCAO animals and quercetin+MCAO animals, respectively (Figure 3B). We used immunofluorescence staining technique to visualize the effect of quercetin in γ-enolase expression on brain tissue sections (Figure 4A). The number of γ-enolase positive cells was significantly decreased in the ischemic core of cerebral cortex in vehicle+MCAO animals compared with that of vehicle+sham animals. However, the decrease of γ-enolase positive cells was improved in the ischemic core of cerebral cortex in quercetin+MCAO animals. The number of γ-enolase positive cells was similar in both vehicle- and quercetin-treated animals with sham operation. The ratio of γ-enolase positive cells to DAPI positive cells was 0.13±0.02 and 0.49±0.04 in vehicle+MCAO animals and quercetin+MCAO animals, respectively (Figure 4B).
Quercetin has a neuroprotective effect in ischemic stroke and protects brain tissues from MCAO-induced neuronal cell injury [824]. Quercetin treatment remarkably reduces the infarct volume in a cerebral ischemia animal model and prevents neuronal cell death [25]. Quercetin administration during the acute phase of brain ischemia significantly induces the expression of antioxidants and reinstates the mitochondrial functions, consequently prevents the cell death [2627]. This study elucidated the regulation of γ-enolase by quercetin in focal cerebral ischemia.
Enolases are very important for energy generation during glycolysis and the deterioration in enolase activity adversely affects the process of energy metabolism in brain. The overexpression of enolases promotes the growth of cultured neuronal tissues [2829]. Moreover, enolase enhances neuronal survival and regenerates axonal growth, and consequently acts as a neurotrophic agent [2526]. The down regulation of enolase leads to neurodegeneration and γ-enolase has been demonstrated as a stress marker for neuronal diseases [30]. This study showed that γ-enolase expression has declined after MCAO operation, while quercetin treatment prevents the injury-induced decrease of γ-enolase. These results were confirmed by several experimental techniques including Western blot, reverse transcription-PCR, and immunofluorescence staining. γ-Enolase is a neurotrophic factor and promotes neuronal differentiation and neurite regeneration [929]. γ-Enolase triggers the activation of PI3K/Akt pathways and leads to neuronal cell survival [31]. It is reported that quercetin attenuates cell apoptosis in focal cerebral ischemia via the activation of PI3K/Akt signaling pathway [832]. Moreover, quercetin enhances exercise-mediated functional recovery after brain ischemia through up-regulation of PI3K/Akt activity and promotion of anti-oxidative and anti-apoptotic signaling pathways [33]. Our results clearly showed that quercetin modulates the expression level of γ-enolase in MCAO-induced ischemic brain injury. However, further studies are needed to elicit the biochemical relation between quercetin and γ-enolase expression. Our findings in this study suggest that quercetin attenuates the γ-enolase reduction in ischemic brain insult and consequently prevents the neuronal cell death through the neuroprotective mechanism of quercetin. In conclusion, we propose that quercetin treatment in cerebral ischemia modulates the expression of γ-enolase and this action mediated by quercetin might be one of the neuroprotective mechanisms contributing to neuronal cell survival.
Figures and Tables
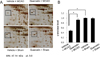 | Figure 1γ-Enolase protein spots identified by MALDI-TOF in the vehicle+sham, quercetin+sham, vehicle+middle cerebral artery occlusion (MCAO), and quercetin+MCAO animals. Squares indicate the γ-enolase protein spots (A). The intensity of spots was measured using PDQuest software (B). The ratio of intensity is described as spots intensity of these animals to spots intensity of sham+vehicle animals. Data (n=4) are shown as mean±SEM. *P<0.05. |
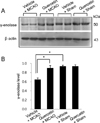 | Figure 2Western blot analysis of γ-enolase protein levels in the vehicle+sham, quercetin+sham, vehicle+middle cerebral artery occlusion (MCAO), and quercetin+MCAO animals. Each lane represents an individual animal (A). Densitometric analysis is represented as intensity of γ-enolase to intensity of β-actin (B). Data (n=4) are shown as mean±SEM. *P<0.05. |
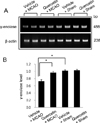 | Figure 3Reverse transcription-PCR analysis of γ-enolase protein levels in the vehicle+sham, quercetin+sham, vehicle+middle cerebral artery occlusion (MCAO), and quercetin+MCAO animals. Each lane represents an individual animal (A). Densitometric analysis is represented as intensity of γ-enolase to intensity of β-actin (B). Data (n=4) are shown as mean±SEM. *P<0.05. |
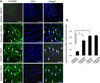 | Figure 4Images of double immunofluorescence labeling with γ-enolase (green color) and DAPI (nuclei marker, blue) in the ischemic core of cerebral cortex in vehicle+sham, quercetin+ sham, vehicle+middle cerebral artery occlusion (MCAO), and quercetin+MCAO animals (A). Quantitative assessment of γ-enolase positive neurons in ischemic core of rat cerebral cortex (B). The arrows indicate the γ-enolase positive cells. Data (n=4) are shown as mean±SEM.*P<0.05. Scale bars=100 µm. |
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2015R1D1A1A01058270).
References
1. Cho JY, Kim IS, Jang YH, Kim AR, Lee SR. Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci Lett. 2006; 404(3):330–335.
2. Dok-Go H, Lee KH, Kim HJ, Lee J, Song YS, Lee YH, Jin C, Lee YS, Cho J. Neuroprotective effects of antioxidative flavonoids, quercetin, dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntiaficus-indica var. saboten. Brain Res. 2003; 965(1-2):130–136.
3. Hajieva P, Bayatti N, Granold M, Behl C, Moosmann B. Membrane protein oxidation determines neuronal degeneration. J Neurochem. 2015; 133(3):352–367.
4. Heo HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004; 52(25):7514–7517.
5. Mahesh T, Menon VP. Quercetin alleviates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res. 2004; 18(2):123–127.
6. Fiorani M, De Sanctis R, Menghinello P, Cucchiarini L, Cellini B, Dachà M. Quercetin prevents glutathione depletion induced by dehydroascorbic acid in rabbit red blood cells. Free Radic Res. 2001; 34(6):639–648.
7. Bate C, Salmona M, Williams A. Ginkgolide B inhibits the neurotoxicity of prions or amyloid-beta1-42. J Neuroinflammation. 2004; 1(1):4.
8. Ahmad A, Khan MM, Hoda MN, Raza SS, Khan MB, Javed H, Ishrat T, Ashafaq M, Ahmad ME, Safhi MM, Islam F. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem Res. 2011; 36(8):1360–1371.
9. Schmechel DE, Brightman MW, Marangos PJ. Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res. 1980; 190(1):195–214.
10. Ueta H, Nagasawa H, Oyabu-Manabe Y, Toida K, Ishimura K, Hori H. Localization of enolase in synaptic plasma membrane as an alphagamma heterodimer in rat brain. Neurosci Res. 2004; 48(4):379–386.
11. Rider CC, Taylor CB. Enolase isoenzymes in rat tissues. Electrophoretic, chromatographic, immunological and kinetic properties. Biochim Biophys Acta. 1974; 365(1):285–300.
12. Lamandé N, Mazo AM, Lucas M, Montarras D, Pinset C, Gros F, Legault-Demare L, Lazar M. Murine muscle-specific enolase: cDNA cloning, sequence, and developmental expression. Proc Natl Acad Sci U S A. 1989; 86(12):4445–4449.
13. Schmechel D, Marangos PJ, Zis AP, Brightman M, Goodwin FK. Brain enolases as specific markers of neuronal and glial cells. Science. 1978; 199(4326):313–315.
14. Marangos PJ, Schmechel D, Parma AM, Clark RL, Goodwin FK. Measurement of neuron-specific (NSE) and non-neuronal (NNE) isoenzymes of enolase in rat, monkey and human nervous tissue. J Neurochem. 1979; 33(1):319–329.
15. Meric E, Gunduz A, Turedi S, Cakir E, Yandi M. The prognostic value of neuron-specific enolase in head trauma patients. J Emerg Med. 2010; 38(3):297–301.
16. González-García S, González-Quevedo A, Fernández-Concepción O, Peña-Sánchez M, Menéndez-Saínz C, Hernández-Díaz Z, Arteche-Prior M, Pando-Cabrera A, Fernández-Novales C. Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem. 2012; 45(16-17):1302–1307.
17. Hay E, Royds JA, Davies-Jones GA, Lewtas NA, Timperley WR, Taylor CB. Cerebrospinal fluid enolase in stroke. J Neurol Neurosurg Psychiatry. 1984; 47(7):724–729.
18. Cronberg T, Rundgren M, Westhall E, Englund E, Siemund R, Rosén I, Widner H, Friberg H. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology. 2011; 77(7):623–630.
19. Steinhoff BJ, Tumani H, Otto M, Mursch K, Wiltfang J, Herrendorf G, Bittermann HJ, Felgenhauer K, Paulus W, Markakis E. Cisternal S100 protein and neuron-specific enolase are elevated and site-specific markers in intractable temporal lobe epilepsy. Epilepsy Res. 1999; 36(1):75–82.
20. Pu F, Mishima K, Irie K, Motohashi K, Tanaka Y, Orito K, Egawa T, Kitamura Y, Egashira N, Iwasaki K, Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007; 104(4):329–334.
21. Dong YS, Wang JL, Feng DY, Qin HZ, Wen H, Yin ZM, Gao GD, Li C. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci. 2014; 11(3):282–290.
22. Li X, Wang H, Gao Y, Li L, Tang C, Wen G, Yang Y, Zhuang Z, Zhou M, Mao L, Fan Y. Quercetin induces mitochondrial biogenesis in experimental traumatic brain injury via the PGC-1α signaling pathway. Am J Transl Res. 2016; 8(8):3558–3566.
23. Lin X, Lin CH, Zhao T, Zuo D, Ye Z, Liu L, Lin MT. Quercetin protects against heat stroke-induced myocardial injury in male rats: Antioxidative and antiinflammatory mechanisms. Chem Biol Interact. 2017; 265:47–54.
24. Ghosh A, Sarkar S, Mandal AK, Das N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS One. 2013; 8(4):e57735.
25. Annapurna A, Ansari MA, Manjunath PM. Partial role of multiple pathways in infarct size limiting effect of quercetin and rutin against cerebral ischemia-reperfusion injury in rats. Eur Rev Med Pharmacol Sci. 2013; 17(4):491–500.
26. Rogerio AP, Kanashiro A, Fontanari C, da Silva EV, Lucisano-Valim YM, Soares EG, Faccioli LH. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007; 56(10):402–408.
27. Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer's disease. J Nutr Biochem. 2009; 20(4):269–275.
28. Takei N, Kondo J, Nagaike K, Ohsawa K, Kato K, Kohsaka S. Neuronal survival factor from bovine brain is identical to neuron-specific enolase. J Neurochem. 1991; 57(4):1178–1184.
29. Hattori T, Takei N, Mizuno Y, Kato K, Kohsaka S. Neurotrophic and neuroprotective effects of neuron-specific enolase on cultured neurons from embryonic rat brain. Neurosci Res. 1995; 21(3):191–198.
30. Hafner A, Obermajer N, Kos J. γ-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem J. 2012; 443(2):439–450.
31. Parnetti L, Palumbo B, Cardinali L, Loreti F, Chionne F, Cecchetti R, Senin U. Cerebrospinal fluid neuron-specific enolase in Alzheimer's disease and vascular dementia. Neurosci Lett. 1995; 183(1-2):43–45.
32. Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res. 2012; 37(12):2777–2786.
33. Chang HC, Yang YR, Wang PS, Wang RY. Quercetin enhances exercise-mediated neuroprotective effects in brain ischemic rats. Med Sci Sports Exerc. 2014; 46(10):1908–1916.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download