This article has been
cited by other articles in ScienceCentral.
Abstract
Successful male germ cell transplantation requires depletion of the host germ cells to allow efficient colonization of the donor spermatogonial stem cells. Although a sterilizing drug, busulfan, is commonly used for the preparation of recipient models before transplantation, the optimal dose of this drug has not yet been defined in dogs. In this study, 1-year-old mongrel dogs were intravenously injected with three different concentrations of busulfan (10, 15, or 17.5 mg/kg). Four weeks after busulfan treatment, no fully matured spermatozoa were detected in any of the busulfan-treated groups. However, small numbers of PGP9.5-positive spermatogonia were detected in all treatment groups, although no synaptonemal complex protein-3-positive spermatocytes were detected. Of note, acrosin-positive spermatids were not detected in the dogs treated with 15 or 17.5 mg/kg busulfan, but were detected in the other group. Eight weeks after busulfan treatment, the dogs treated with 10 mg/kg busulfan fully recovered, but those in the other groups did not. PGP9.5-positive spermatogonia were detected in the 10 mg/kg group, and at a similar level as in the control group, but these cells were rarely detected in the 15 and 17.5 mg/kg groups. These results suggest that a dose of 15-17.5 mg/kg is optimal for ablative treatment with busulfan to prepare the recipient dogs for male germ cell transplantation. At least eight weeks should be allowed for recovery. The results of this study might facilitate the production of recipient dogs for male germ cell transplantation and can also contribute to studies on chemotherapy.
Go to :

Keywords: Dog, busulfan, testis, germ cell
Spermatogenesis is a complex process by which spermatogonial stem cells (SSCs) in the seminiferous tubules of an adult testis become mature spermatozoa. SSCs can be transplanted into the seminiferous tubules of sterile recipient testes to provide an effective strategy to study spermatogenesis and the production of transgenic animals [
1]. The preparation of a completely sterile recipient is key to producing donor-derived sperm using SSC transplantation [
2]. Several methods have been developed in mammals, such as the use of irradiation, chemical reagents, surgical cryptorchidism, and heat therapy [
1345].
Busulfan treatment is the most commonly used method for chemical sterilization. Busulfan is a DNA alkylating agent that depletes germ cells and disrupts the junctions between Sertoli cells, thereby permitting the migration of transplanted spermatogonia to the basal lamina [
6]. Several studies have reported the production of recipient testes for male germ cell transplantation. The dose of busulfan required for sterilization varies widely between species. Doses of 30-44 mg/kg have been reported in mice, 40-100 mg/kg in pigs, and 8-12 mg/kg in monkeys [
789]. In dogs, busulfan was previously intravenously injected at doses ranging from 3.75 to 40 mg/kg [
10]. However, the previous study did not analyze germ cell depletion after the treatment, so the optimal dose for dogs is currently unclear.
In this study, we examined the dose-dependent effects of busulfan treatment and performed a histological analysis of busulfan-treated dog testes. In addition, biomarkers of spermatogonia, spermatocytes, and spermatid cells were analyzed by immunohistochemistry.
Materials and Methods
Animals and experimental design
One-year-old mongrel dogs (n=10, about 15-20 kg) were randomly divided into four groups: control (Con, n=1), 10 mg/kg (n=3), 15 mg/kg (n=3), and 17.5 mg/kg (n=3). Busulfan (Sigma Aldrich, B2635) was dissolved in dimethyl sulfoxide and injected into the vein with 50 mL of saline. Testicular tissues were collected four and eight weeks after busulfan treatment by a veterinary surgeon using a Vitesse Biopsy Gun (OptiMed global care, 1399-000) after anesthesia. Each sample was fixed in Bouin's solution overnight at 4℃. The changes in body weight were measured every week. Experimental procedures were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of the National Institute of Animal Science (approval No. 2016-214).
Histology
Fixed testes samples were subsequently washed with 70-100% (v/v) ethanol, washed in xylene, embedded in paraffin, sliced into 5-µm-thick sections using a microtome (Thermo Fisher Scientific, Waltham, MA, USA), and mounted on glass slides. The mounted dog testicular tissue was rehydrated with xylene and a gradient of 100-0% ethanol. The rehydrated testes specimens were stained with hematoxylin and eosin (H&E).
Immunohistochemistry
To identify the localization of PGP9.5-, SCP3- and acrosin-expressing cells in the dog testes, the antigens were retrieved by boiling the tissue for 30 minutes in Tris-ethylenediaminetetraacetic acid (Tris-EDTA) solution (10 mM Tris base, 1 mM EDTA, and 0.05% Tween-20; pH 9). The membranes of the antigen-retrieved tissues were permeabilized with phosphate-buffered saline (PBS) containing 0.2% Triton X-100 for 10 minutes. Non-specific protein binding was blocked with 2% bovine serum albumin in PBS for 1 hour at room temperature (RT). Tissues were incubated overnight at 4℃ with the following primary antibodies: PGP9.5 (1:1000 dilution; 7863-1004; AbD Serotec; Raleigh, NC, USA), SCP3 (1:50 dilution; SC-33195; Santa Cruz Biotechnology, Inc.) and acrosin (1:50 dilution; SC-51504; Santa Cruz Biotechnology, Inc.). After the samples were washed three times with PBS, the appropriate secondary antibody was added and the samples were incubated for 2 hours at RT. A peroxidase substrate detection kit (SK-4100; Vector Laboratories; Burlingame, CA, USA) was used for the detection of putative canine germ cell markers, according to the manufacturer's instructions. Finally, DPX mountant (Sigma Aldrich, 06522) was used to fix the immunostained canine testicular tissues. The immunostained tissues were observed under an optical microscope.
Go to :

Results
Changes in body weight after busulfan treatment
To determine the side effects of busulfan treatment, the dogs' body weight was measured. There was an increase in body weight in all of the dogs (
Table 1). However, one dog each in the 15 and 17.5 mg/kg treatment groups died within the first 15 and 18 days after busulfan treatment.
Table 1
Changes in the body weight of dogs following busulfan treatment
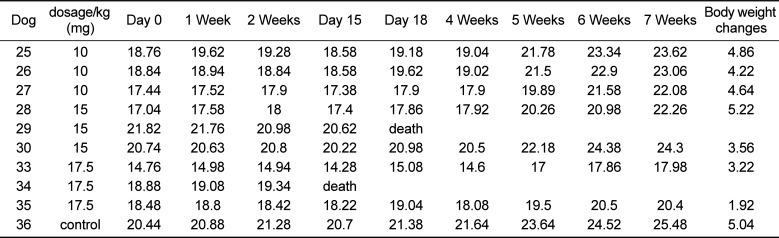
|
Dog |
dosage/kg (mg) |
Day 0 |
1 Week |
2 Weeks |
Day 15 |
Day 18 |
4 Weeks |
5 Weeks |
6 Weeks |
7 Weeks |
Body weight changes |
|
25 |
10 |
18.76 |
19.62 |
19.28 |
18.58 |
19.18 |
19.04 |
21.78 |
23.34 |
23.62 |
4.86 |
|
26 |
10 |
18.84 |
18.94 |
18.84 |
18.58 |
19.62 |
19.02 |
21.5 |
22.9 |
23.06 |
4.22 |
|
27 |
10 |
17.44 |
17.52 |
17.9 |
17.38 |
17.9 |
17.9 |
19.89 |
21.58 |
22.08 |
4.64 |
|
28 |
15 |
17.04 |
17.58 |
18 |
17.4 |
17.86 |
17.92 |
20.26 |
20.98 |
22.26 |
5.22 |
|
29 |
15 |
21.82 |
21.76 |
20.98 |
20.62 |
death |
|
|
|
|
|
|
30 |
15 |
20.74 |
20.63 |
20.8 |
20.22 |
20.98 |
20.5 |
22.18 |
24.38 |
24.3 |
3.56 |
|
33 |
17.5 |
14.76 |
14.98 |
14.94 |
14.28 |
15.08 |
14.6 |
17 |
17.86 |
17.98 |
3.22 |
|
34 |
17.5 |
18.88 |
19.08 |
19.34 |
death |
|
|
|
|
|
|
|
35 |
17.5 |
18.48 |
18.8 |
18.42 |
18.22 |
19.04 |
18.08 |
19.5 |
20.5 |
20.4 |
1.92 |
|
36 |
control |
20.44 |
20.88 |
21.28 |
20.7 |
21.38 |
21.64 |
23.64 |
24.52 |
25.48 |
5.04 |

Histological changes induced by busulfan treatment
To analyze the effects of busulfan on the dog testes, H&E staining was performed. No fully-differentiated spermatozoa were detected in any of the groups (
Figure Ab, Ac, Ad). However, some differentiated cells were detected in the dogs treated with 10 mg/kg busulfan. In addition, spermatozoa were detected eight weeks after treatment in the 10 mg/kg busulfan group, but not in the 15 and 17.5 mg/kg treatment groups. Moreover, the diameter of the seminiferous tubules had recovered to normal in the 10 mg/kg treatment group (
Figure 1. Bb).
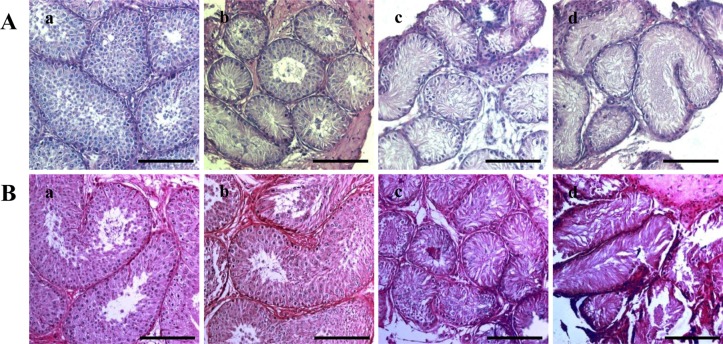 | Figure 1Hematoxylin and eosin (H&E) staining of dog testes after busulfan treatment. H&E staining was performed four (A) and eight (B) weeks after treatment on testes from dogs in the (a) control, (b) 10 mg/kg, (c) 15 mg/kg and (d) 17.5 mg/kg groups. The scale bars indicate 100 µm.
|
Effects of busulfan treatment on the expression of germ cell markers
Immunohistochemistry was performed to assess the testicular expression of PGP9.5, a marker of undifferentiated spermatogonia, and SCP3 and acrosin, markers of differentiated male germ cells. The expression of PGP9.5 significantly decreased four weeks after busulfan injection (
Figure 2. A). However, after eight weeks, the number of PGP9.5-positive cells had recovered in the dogs treated with 10 mg/kg busulfan, although this recovery was not seen in the 15 mg/kg and 17.5 mg/kg groups (
Figure 2. Bb, Bc, Bd). No SCP3 expression (as a marker of spermatocytes) was detected in either group at four or eight weeks after busulfan injection (
Figure 3. Ab, Ac, Ad, Bb, Bc, Bd). The expression of acrosin was not detected at four or eight weeks after injection in either the 15 or 17.5 mg/kg busulfan groups (
Figure 4. Ac, Ad, Bc, Bd). However, while the number of acrosin-expressing cells significantly decreased at four weeks in the 10 mg/kg busulfan group, the cells had recovered by eight weeks after busulfan injection (
Figure 4. Ab, Bb).
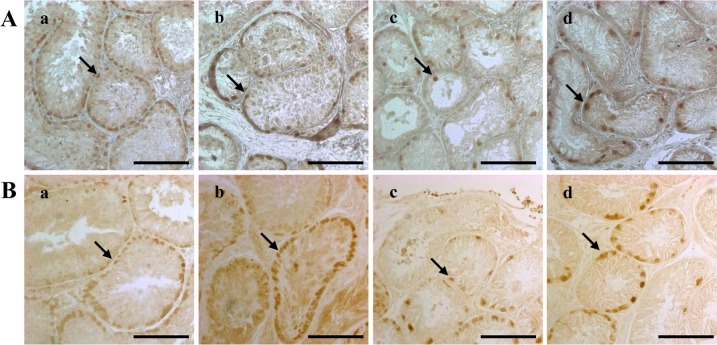 | Figure 2Dog spermatogonia as detected by PGP9.5 immunohistochemical staining. The staining was performed four (A) and eight (B) weeks after treatment on testes from dogs in the (a) control, (b) 10 mg/kg, (c) 15 mg/kg and (d) 17.5 mg/kg groups. The scale bars indicate 100 µm. The arrows indicate cells expressing the PGP9.5 protein. PGP9.5, protein gene product 9.5.
|
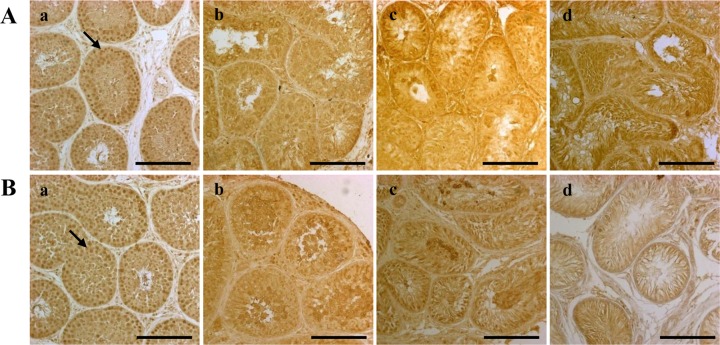 | Figure 3Dog spermatocytes, as detected by SCP3 immunohistochemical staining. The staining was performed four (A) and eight (B) weeks after treatment on testes from dogs in the (a) control, (b) 10 mg/kg, (c) 15 mg/kg and (d) 17.5 mg/kg groups. The scale bars indicate 100 µm. The arrows indicate cells expressing the SCP3 protein. SCP3, synaptonemal complex protein 3.
|
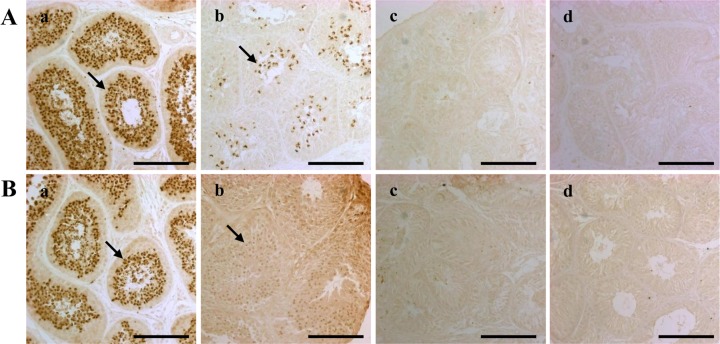 | Figure 4Dog spermatids and spermatozoa as detected by acrosin immunohistochemical staining. The staining was performed four (A) and eight (B) weeks after treatment on testes from dogs in the (a) control, (b) 10 mg/kg, (c) 15 mg/kg and (d) 17.5 mg/kg groups. The scale bars indicate 100 µm. The arrows indicate cells expressing the acrosin protein.
|
Go to :

Discussion
We investigated the histological and cellular changes in dog testes following busulfan treatment to establish sterile males to serve as germ cell transplant recipients. Our previous study reported that PGP9.5 was detected in undifferentiated spermatogonia, SCP3 was detected in primary spermatocytes, and acrosin was detected in the spermatids and spermatozoa in dog testes [
11]. We therefore also analyzed the expression of these markers in dog testes after busulfan treatment.
Male germ cell transplantation is a useful technology to conserve endangered species and produce transgenic animals [
12]. To prepare the recipient testes for male germ cell transplantation, it is necessary to achieve maximal depletion of endogenous germ cells with minimal effects on the testicular microenvironment [
13]. Treatment with busulfan is the most common method used for germ cell depletion. Several studies have described the effects of busulfan treatment for the preparation of recipient testes, but data are still lacking for dogs. Although one previous study analyzed the dose-dependent effects of busulfan in dogs, that report did not describe germ cell depletion and recovery in the dogs [
10].
Busulfan, an alkylating agent that is toxic to stem cells, is used to condition patients for autologous and allogeneic hematopoietic stem cell transplantation [
14]. It has also been used experimentally in animals for germ cell transplantation because it can deplete germ cells and disrupt the junctions between Sertoli cells, thus permitting the in dog testes after busulfan treatment.
Male germ cell transplantation is a useful technology to conserve endangered species and produce transgenic animals [
12]. To prepare the recipient testes for male germ cell transplantation, it is necessary to achieve maximal depletion of endogenous germ cells with minimal effects on the testicular microenvironment [
13]. Treatment with busulfan is the most common method used for germ cell depletion. Several studies have described the effects of busulfan treatment for the preparation of recipient testes, but data are still lacking for dogs. Although one previous study analyzed the dose-dependent effects of busulfan in dogs, that report did not describe germ cell depletion and recovery in the dogs [
10].
Busulfan, an alkylating agent that is toxic to stem cells, is used to condition patients for autologous and allogeneic hematopoietic stem cell transplantation [
14]. It has also been used experimentally in animals for germ cell transplantation because it can deplete germ cells and disrupt the junctions between Sertoli cells, thus permitting the migration of transplanted spermatogonia [
6]. In the present study, male germ cells were depleted in dog testes by busulfan treatment. We noted that a dose of 10 mg/kg busulfan is insufficient for recipient testis preparation (
Figure 1). However, both 15 and 17.5 mg/kg busulfan depleted the male germ cells, and the cells remained depleted for at least eight weeks after treatment in the dog testes (
Figure 1).
In this study, histological analysis of seminiferous tubule showed reversible loss of spermatogenic cells. Previous report described, mice that received a lower dosage of busulfan may have some tubule repopulation from endogenous spermatogonial stem cells. The count of germ cell in the groups treated with busulfan after 8 weeks, showed a considerable recovery of spermatogenesis [
15].
Biomarkers of male germ cells are important for research on spermatogenesis. In this study, PGP9.5, SCP3, and acrosin proteins were used to identify germ cells at different stages of spermatogenesis. The numbers of PGP9.5-positive undifferentiated spermatogonia were significantly reduced by busulfan treatment (
Figure 2), and recovered in the 10 mg/kg-treated testes by eight weeks after treatment. This result indicates that 10 mg/kg of busulfan was not sufficient for preparation of the recipient testis. Interestingly, the SCP3 protein was not detected and the acrosin protein was only weakly detected after busulfan treatment (
Figure 3,
4). These results indicate that busulfan leads to relatively long-term damage to the testes after injection. These results need to be confirmed in other studies, but suggest that more research is needed to determine the potential for spermatogenic recovery after chemotherapy.
In conclusion, we analyzed the effects of busulfan treatment on dog testis for up to eight weeks after injection. Our findings indicate that 10 mg/kg is not sufficient for germ cell depletion. Both 15 and 17.5 mg/kg treatment can deplete germ cells in dog testes, but not perfectly. Nevertheless, a dose of 15-17.5 mg/kg and eight weeks of recovery are optimal for busulfan treatment to prepare recipient dogs for male germ cell transplantation.
Go to :

Acknowledgments
This study was supported by grant (PJ01186502) from the Cooperative Research Program for Agriculture Science & Technology Development, Rural Development Administration, Republic of Korea.
Go to :

Notes
Go to :

References
1. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994; 91(24):11298–11302. PMID:
7972053.

2. Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999; 31(5):461–472. PMID:
10612257.

3. Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M, Schlatt S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011; 26(8):1945–1954. PMID:
21613315.

4. Shinohara T, Avarbock MR, Brinster RL. Functional analysis of spermatogonial stem cells in Steel and cryptorchid infertile mouse models. Dev Biol. 2000; 220(2):401–411. PMID:
10753526.

5. Jegou B, Peake RA, Irby DC, de Kretser DM. Effects of the induction of experimental cryptorchidism and subsequent orchidopexy on testicular function in immature rats. Biol Reprod. 1984; 30(1):179–187. PMID:
6141812.
6. Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res. 1987; 176(2):259–268. PMID:
3807936.

7. Wang DZ, Zhou XH, Yuan YL, Zheng XM. Optimal dose of busulfan for depleting testicular germ cells of recipient mice before spermatogonial transplantation. Asian J Androl. 2010; 12(2):263–270. PMID:
20010847.

8. Kim JH, Jung-Ha HS, Lee HT, Chung KS. Development of a positive method for male stem cell-mediated gene transfer in mouse and pig. Mol Reprod Dev. 1997; 46(4):515–526. PMID:
9094099.

9. Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, Peterson K, Masterson K, Ramsey C, Ward T, Lienesch M, Volk A, Cooper DK, Thomson AW, Kiss JE, Penedo MC, Schatten GP, Mitalipov S, Orwig KE. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012; 11(5):715–726. PMID:
23122294.

10. Deeg HJ, Schuler US, Shulman H, Ehrsam M, Renner U, Yu C, Storb R, Ehninger G. Myeloablation by intravenous busulfan and hematopoietic reconstitution with autologous marrow in a canine model. Biol Blood Marrow Transplant. 1999; 5(5):316–321. PMID:
10534062.

11. Lee WY, Lee R, Park HJ, Do JT, Park C, Kim JH, Jhun H, Lee JH, Hur T, Song H. Characterization of male germ cell markers in canine testis. Anim Reprod Sci. 2017; 182:1–8. See comment in PubMed Commons below. PMID:
28465081.

12. Hill JR, Dobrinski I. Male germ cell transplantation in livestock. Reprod Fertil Dev. 2006; 18(1-2):13–18. PMID:
16478598.

13. Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003; 69(2):412–420. PMID:
12672656.
14. Hassan M, Oberg G, Björkholm M, Wallin I, Lindgren M. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemother Pharmacol. 1993; 33(3):181–186. PMID:
8269597.

15. Boettger-Tong HL, Johnston DS, Russell LD, Griswold MD, Bishop CE. Juvenile spermatogonial depletion (jsd) mutant seminiferous tubules are capable of supporting transplanted spermatogenesis. Biol Reprod. 2000; 63(4):1185–1191. PMID:
10993844.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download