Abstract
Recently, worldwide dietary reference intakes have been considered an important guideline for public health. Some governments and the World Health Organization (WHO) provide guidelines concerning dietary intake. Although an ingredient may have a history of use as a culinary material, changes in the environment over time suggest that the acceptable maximum intake each of food/culinary material should be regularly evaluated. Arctium lappa L. has been used as a culinary material for many centuries in Korea and Japan and some recent studies have reported related therapeutic effects. However, there are no reports on the safety of repeated oral administration. In this study, we evaluated the safety of a 8-weeks repeated oral intake of A. lappa. We concluded that treatment with <250 mg/kg A. lappa, which was within the safety range, resulted in body weight decrease and blood glucose suppression.
Currently, the nutritional aspects of excessive eating in developed countries are contrasted by severe problems, such as the scarcity of food, in developing countries. Many nations and the World Health Organization (WHO) provide guidelines for reference dietary intakes [1234] to assist in public health. In 2013, the Ministry of Health and Welfare in Korea [1] provided dietary references for food intake that included a majority of the foods in Korean society. The guidelines contain not only a “Recommended Nutrient Intake” but also a “Tolerable Upper Intake Level” (UL). The UL is important, as it indicates that overconsumption of a nutritional food may lead to health problems. Although an ingredient may have been previously used as a culinary material, environmental changes that occur over time require that further safety studies for these ingredients should be conducted.
Arctium lappa L. has been used as a culinary material for centuries, but its recently reported bioactivities have included the suppression of renal interstitial fibrosis in obstructive nephropathy [5], a protective effect against ethanol-induced neurotoxicity [6], and gastroprotective [7] and anti-metastatic [8] effects.
However, there are no data for the safety of repeated oral intake and therefore we evaluated the safety of a 8-weeks repeated oral administration of A. lappa and the potential reduction of blood glucose levels.
Arctium lappa was purchased in October 2016 from a traditional market in Naju-si Jeonnam province, Korea. A voucher specimen (DSUOB-AL-01) was deposited at the College of Oriental Medicine, Dongshin University. The root was separated for the study and air-dried. The powdered A. lappa roots (250 g) were extracted twice with hot water (1 L) at room temperature for 3 days. After filtration, the water was evaporated, freeze dried, and stored at −50℃. The crude extract was resuspended in distilled water and filtered through a 0.4-µm membrane. The yield of A. lappa roots extract with hot water was 5%.
In order to evaluate the safety of A. lappa, 32 C57BL/6 mice were purchased from SamTako BioKorea (Osan, Korea) and acclimatized to experimental conditions for 7 days. The mice were divided into four treatment groups and treated for 8-weeks with ad libitum access to the relevant diet and water: (1) control; (2) 60% fat diet; (3) 60%-fat diet and 50 mg/kg/day A. lappa treatment; and (4) 60%-fat diet and 250 mg/kg/day A lappa treatment. The 60% fat-diet was provided by Research Diets, Inc. (Product #D12492, New Brunswick, NJ, USA; Table 2). All experiments were approved by the Institutional Animal Care and Use Committee at Chonnam National University (Approval No. CNU IACUC-YB-R-2016-50).
All animals were observed twice per day for physiological changes, such as movement, fur color/grooming, appetite, aggression, and body weight and dietary consumption was measured twice per week. At 3 h before sacrifice, feeding was restricted and the rats were anesthetized with intraperitoneal injections of 50 mg/kg Zoletil (Virbac, Fort Worth, TX, USA). After sacrifice, the appearance of all organs was observed with the naked eye to identify any toxicological changes. Whole blood was collected from the heart and analyzed with a Hemavet Multispecies Hematology System (Drew Scientific Inc., Waterbury, CT, USA) (n=8 per group). Five organs (heart, lung, kidney, brain, and spleen) were collected for the evaluation of the morphological changes that result from the treatment with A. lappa. All organs were fixed in 10% (v/v) formaldehyde solution, dehydrated in a graded ethanol series (99.9, 90, 80, and 70%), and embedded in paraffin. The paraffin-embedded lung tissue was then sectioned (4 µm) longitudinally and stained with hematoxylin and eosin.
After acclimatization for 7 days, all mice were weighed and the mean body weight in each group was adjusted from 22.1±1.66 g in order to standardize the differences in body weight among each group (Figure 1). In the control group, the mice were fed a normal diet and the body weight increase was approximately 6.7 g; in comparison, the body weight of animals fed a 60%-fat diet had increased by approximately 15.8 g after 8-weeks. Although the body weight gain was the lowest in the control group, among the 60%-fat diet-fed groups, the mean body weight in the 250 mg/kg/day A. lappa-treated group was 33.7±2.96 g.
The comparison of the white blood cell (WBC)/differential cell counts in the control group in indicated a significant increase in the high-fat diet groups (Table 1). The number of WBC in the control group was 16.6±8.32×102 cells/mL and in the 60%-fat diet group it was 52.4±18.36×102 cells/mL. The number of WBC in the control group three times higher than that of the 60%-fat diet group. However, there was no difference between the animals fed the 60-fat diet and those fed the 60%-fat diet and treated with A. lappa. This result indicated that A. lappa treatment under 250 mg/kg/day for 8-weeks did not affect the changes in WBC. The results of the differential cell counts were similar to those of WBC. Although each cell involving WBC was increased by the 60% fat diet there, was no change between the 60%-fat diet and those fed the 60% fat-diet and treated with A. lappa. The results indicated that A. lappa L. treatment did not influence the changes of each cell in WBC.
However, the increased blood glucose level that was observed in the 60%-fat diet group was dose-dependently suppressed by A. lappa treatment. The blood glucose level in the 60%-fat diet group was approximately two times higher (474±41 mg/dL) compared with the control group (241±32 mg/dL), but the levels were 341±41 mg/dL in with 60%-fat diet and 50 mg/kg A. lappa treatment and 230±41 mg/dL with 60% -fat diet and 250 mg/dL A. lappa treatment. Thus, A. lappa treatment significantly suppressed the increase in blood glucose.
Many blood glycemic controllers have been reported as culinary ingredients and/or traditional medicine materials, such as Aloe vera [9], bilberry (Vaccinium myrtillus) [10], Okra (Abelmoschus esculentus) [11], and Cinnamon sp. [12]. However, most of their blood glucose suppressive effects were induced by extracts, not by a single isolated component of the extracts. Recently, biological effects of A. lappa have been reported, including the suppression of renal interstitial fibrosis [5], neuroprotection [6], gastroprotection [7], and anti-metastasis [8]. However, no reports have covered the blood glucose suppression effect and although most of anti-diabetic agents have anti-glucose effect, it is necessary to investigate the mode of action and candidate molecules of glucose suppression.
In order to evaluate the histopathological changes in lung (Figure 2A), heart (Figure 2B), kidney (Figure 2C), brain (Figure 2D), and spleen (Figure 2E), the relevant organs were stained with H&E. No morphological changes related to the A. lappa treatment were found in the organs, except for the liver. All images in the “a” panels in each photograph were considered normal as they were from the animals fed the normal diet. The “b” panels are representative images of the 60%-fat diet group. There was no difference between the control group and the 60%-fat diet group. A. lappa treatment did not induce morphological changes in any of the organs observed.
Many natural products have been used worldwide as a culinary material or medicine for centuries. However, although ingredients may have been previously considered safe, recent toxicological analysis techniques has classified them as toxic. In 2015, Cynanchum auriculatum was distributed as C. wilfordii [13] in Korea, but the U.S. Food & Drug Administration classified C. auriculatum as a poisonous plant based on the research of Han et al. [14]. For hundred years, A. lappa has been used as a culinary ingredient that is boiled down in soy sauce in Korea and Japan and is a frequently ingested food. However, as the toxicity of A. lappa repeated administration had not been previously evaluated, we conducted an assessment of the toxicity of a 8-weeks oral administration of A. lappa. The results indicated that A. lappa was very safe and had therapeutic effects, including the suppression of body weight gain and blood glucose.
Acknowledgment
This research was financially supported by the Ministry of Trade, Industry, and Energy (MOTIE), Korea, under the “Regional Specialized Industry Development Program” supervised by the Korea Institute for Advancement of Technology (KIAT, Project No. R0004124).
Notes
References
1. Ministry of Health and Welfare in Korea. 2010 Dietary reference intakes in Korean. 2013.
2. WHO. Food and Agriculture Organization of the United Nations. Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr Pap. 1998; 66:1–140. PMID: 9743703.
3. WHO. Sugars intake for adults and children. 2015.
4. WHO. Food and Agriculture Organization of the United Nations. United Nations University. Protein and amino acid requirements in human nutrition, Report of a joint FAO/WHO/UNU expert consultation (WHO Technical Report Series 935.). 2007.
5. Li A, Zhang X, Shu M, Wu M, Wang J, Zhang J, Wang R, Li P, Wang Y. Arctigenin suppresses renal interstitial fibrosis in a rat model of obstructive nephropathy. Phytomedicine. 2017; 30:28–41. PMID: 28545667.

6. Huang J, Xiao L, Wei JX, Shu YH, Fang SQ, Wang YT, Lu XM. Protective effect of arctigenin on ethanol-induced neurotoxicity in PC12 cells. Mol Med Rep. 2017; 15(4):2235–2240. PMID: 28260103.

7. Li XM, Miao Y, Su QY, Yao JC, Li HH, Zhang GM. Gastroprotective effects of arctigenin of Arctium lappa L. on a rat model of gastric ulcers. Biomed Rep. 2016; 5(5):589–594. PMID: 27882222.
8. Lou C, Zhu Z, Zhao Y, Zhu R, Zhao H. Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol Rep. 2017; 37(1):179–184. PMID: 27878294.
9. Suksomboon N, Poolsup N, Punthanitisarn S. Effect of Aloe vera on glycaemic control in prediabetes and type 2 diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. 2016; 41(2):180–188. PMID: 27009750.
10. de Mello VD, Lankinen MA, Lindström J, Puupponen-Pimiä R, Laaksonen DE, Pihlajamäki J, Lehtonen M, Uusitupa M, Tuomilehto J, Kolehmainen M, Törrönen R, Hanhineva K. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol Nutr Food Res. 2017; 61(9):

11. Khosrozadeh M, Heydari N, Abootalebi M. The Effect of Abelmoschus Esculentus on Blood Levels of Glucose in Diabetes Mellitus. Iran J Med Sci. 2016; 41(3 Suppl):S63.
12. Costello RB, Dwyer JT, Saldanha L, Bailey RL, Merkel J, Wambogo E. Do Cinnamon Supplements Have a Role in Glycemic Control in Type 2 Diabetes? A Narrative Review. J Acad Nutr Diet. 2016; 116(11):1794–1802. PMID: 27618575.
13. Shin HK, Lee YM. ‘Fake Bitsuo Oh’ surge... Cosumers “10% only real”. Yonhap News. 2015. 4. 22.
14. Han J, Luan DH. Sow abortion caused by feeding Cynanchum auriculatum. Animal Husb. Vet. Med. Xumu yu Shouyi. 1984; 16(6):266.
Figure 1
The body weight changes over 8-weeks. Con, normal diet treatment group (control group); 60% fat diet, 60%-fat diet treatment group; 50 mg/kg Arctium lappa L., 50 mg/kg Arctium lappa L. treatment with 60%-fat diet group; 250 mg/kg Arctium lappa L., 250 mg/kg Arctium lappa L. treatment with 60%-fat diet group. The dots and the bars indicate the average and standard deviation.

Figure 2
Histopathological images from rats in each group. A, photographs of the lung; B, photographs of the heart; C, photographs of the kidney; D, photographs of the brain; E, photographs of the spleen. A, normal diet treatment group (control group); B, 60%-fat diet treatment group; C, 50 mg/kg Arctium lappa L. treatment with 60%-fat diet; D, 250 mg/kg Arctium lappa L. treatment with 60%-fat diet. Scale bar: 100 µm

Table 1
The analysis of blood and blood glucose levels
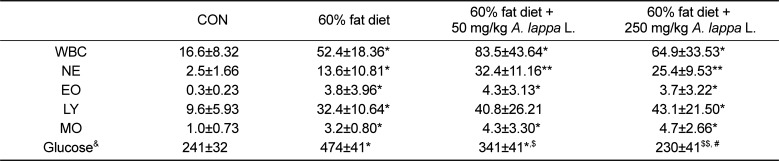
The units of white blood cells and the differential cell count are ×102 cells/mL of whole blood and the unit of blood glucose is ×mg/dL of serum. Unit, ×102 cells/mL; &unit, mg/dL; *vs control group, P<0.01; **vs control group, P<0.005; $vs 60% fat diet treatment, P<0.01; $$vs 60% fat diet treatment, P<0.005; #vs 50 mg/kg Arctium lappa L. treatment with 60% fat diet, P<0.01
Table 2
Formula of 60% fat diet
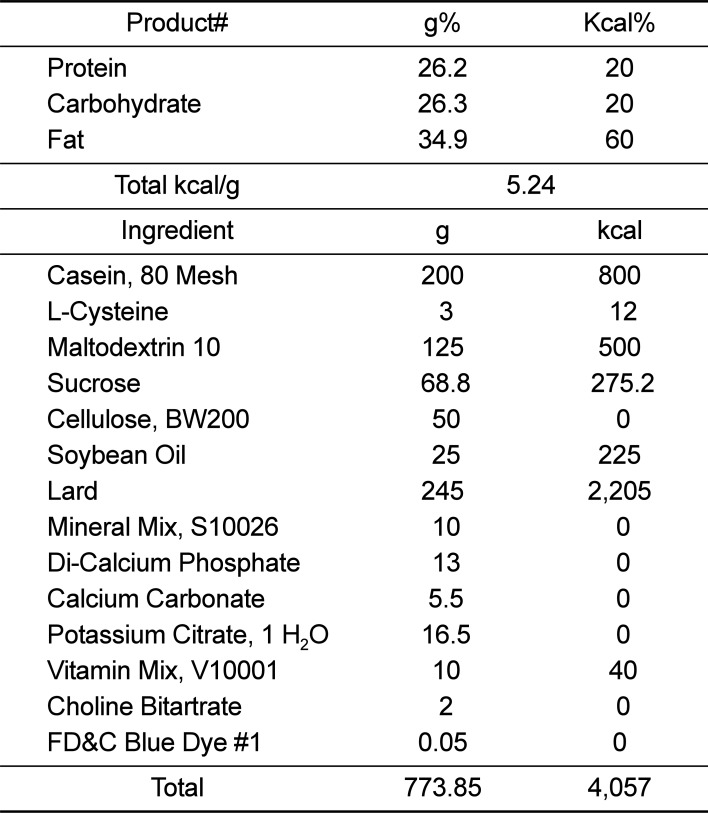
| Product# | g% | Kcal% |
|---|---|---|
| Protein | 26.2 | 20 |
| Carbohydrate | 26.3 | 20 |
| Fat | 34.9 | 60 |
| Total kcal/g | 5.24 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download