Abstract
Purpose
This study aims to evaluate early changes in retinal structure and BMP2 expression in the retina and crystalline lens by comparing streptozotocin-induced diabetic pigs and normal control group pigs.
Methods
Five eye samples from five diabetic Micro-pigs (Medikinetics, Pyeongtaek, Korea) and five eye samples from five control pigs bred in a specific pathogen-free area were used. Diabetes was developed through intravenous injection of nicotinamide and streptozotocin, and the average fasting glucose level was maintained at 250 mg/dL or higher for 16 weeks. To evaluate BMP2 expression in the retina and crystalline lens, Western blotting was performed.
Results
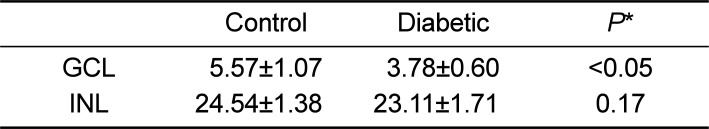
In Hematoxylin and Eosin staining, most diabetic pigs showed structural abnormalities in the inner plexiform layer. The number of nuclei in the ganglion cell layer within the range of 104 µm2 was 3.78±0.60 for diabetic pigs and 5.57±1.07 for control group pigs, showing a statistically significant difference. In immunohistochemical staining, diabetic retinas showed an overall increase in BMP2 expression. In Western blotting, the average BMP2/actin level of diabetic retinas was 1.19±0.05, showing a significant increase compared to the 1.06±0.03 of the control group retinas (P=0.016). The BMP2/actin level of diabetic crystalline lenses was similar to the control group crystalline lenses (P=0.730).
Diabetic retinopathy is a leading cause of vision loss among working aged adults around the world [1]. Previous studies found that thinning of the retina nuclear layer and apoptosis of ganglion cells occur with neurotic regression during the initial stage of diabetes prior to the development of clinically significant angiopathy, such as microaneurysm, exudate, or hemorrhage [23].
Bone morphogenetic protein (BMP) is a protein that enables heterotopic bone formation. First found in bone extract, BMP is important in vascular physiological and pathological terms [4]. Bone Morphogenetic Protein 2 (BMP2), a subgroup of BMP, is involved in secretion during the bone recovery process, plays an essential role in the development of the eye, and is involved in oxidative stress, inflammation, and angiogenesis through the BMP signaling pathway [5]. Recently, a study reported that BMP2 is a potential cause of vascular inflammation such as diabetic retinopathy or other illnesses during the vascular formation process [6]; however, the mechanism behind it remains unclear. There are many merits to using mice in a study on diabetes; however, there are many genetic and physiological differences between mice and humans compared to other mammals such as monkeys or pigs. The retina of a pig, compared to large-sized animals such as dogs, goats, or cows, is relatively similar to that of a human.
The purpose of this study is to evaluate early changes in retinal structure and BMP2 expression levels in the retina, crystalline lens, and vitreous humor in streptozotocin-induced diabetic miniature pigs and normal control pigs.
The subjects of the experiment were male micro-pigs (Medi Kinetics, Pyeongtaek, Korea) bred in a specific pathogen-free area, aged six months with less than 20 kg of body weight [7]. Five eye samples were obtained from five micro-pigs with diabetes, and five eye samples were obtained from five normal control group pigs bred in the same environment. The blood glucose and serum insulin level were followed as shown in previous report [7]. The entire experiment of mini-pigs was done at the animal facility of Medi Kinetics Ltd. All study protocols followed the Guide for Animal Experimental Protocol (MK-IACUC: 2010-0088) under the approval of Institutional Animal Care and Use Committee of Medi Kinetics Co.
The experiment subjects were bred under a controlled environment (18-20 degrees Celsius, 30-70% humidity level, 15 rounds of air change per hour), housed cage individually washed daily, and fed pellets up to 0.5 kg per head per day with free accessible water. Type 2 diabetes was developed in the subjects through the intravenous injection of Nicotinamide (NIA) and Streptozotocin (STZ). A 67 mg/kg NIA solution at a 300 mg/mL concentration was injected into the NIA/STZ and control groups. A 125 mg/kg STZ solution with a concentration level of 62.5 mg/mL was injected into the NIA/STZ group. Blood was collected before feeding and following fasting for 12-16 hours after the NIA/STZ injection in the experiment group and after a 12-16 hour fast in the control group. The glucose level of the control group was maintained at 100 or below, while that of the experimental group showed an average increase of 250 or more.
No clinical signs such as illness, pain and death were observed prior to the experimental endpoint. For ocular surgery, pigs were anesthetized with ketamine (33 mg/kg) and acepromazine (1.1mg/kg). Eyeballs were enucleated 16 weeks after the development of diabetes, and crystalline lens and vitreous humor were separated after limbal incision was performed around the cornea. After the enucleation, the subjects were euthanized with supersaturated solution of KCl injected intravenously.
Subject retinas were fixed in 10% neutral buffered formalin, and 4 µm paraffin-embedded sections were cut horizontally from the retinas for H&E staining. In the retina section, the area 5.0 mm from the optic nerve was micrographed using a BX43 OLYMPUS fluorescent microscope. The average numbers of nuclei in the ganglion cell layer and inner nuclear layer were measured within 104 µm2 in two sections per subject. The reference point and the area of the micrographed region were measured using IS Capture Software (TUCSEN, Fujian, China). To evaluate the expression level of BMP2, immunohistochemical staining was performed using anti-BMP2 antibody (Abcam, Cambridge, UK). ISOLECTIN B4 (Vector Laboratories, Burlingame, CA, US) was used as a blood vessel marker. The retina sections were incubated with Antibody Diluent solution (Invitrogen, Waltham, MA, US) at room temperature for one hour and then were incubated with anti-BMP2 antibody (5 µg/mL) and isolectin B4 (15 µg/mL) at 4℃ for one day. On the next day, the sections were incubated with Griffonia Simplicifolia Lectin I (Vector Laboratories, Burlingame, CA, US) and FITC-labeled Anti-Rabbit IgG (H+L) Antibody. After sealing the sections with 4',6'-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, US), their image was obtained using a BX43 OLYMPUS fluorescence microscope (Olympus, Tokyo, Japan) and IS Capture Software. Evaluation of the stain result was completed by two ophthalmologists through a double-blind method.
Western blotting was conducted to assess the expression of BMP2 in the pig retina and crystalline lens. Using RIPA Buffer (50 mM Tris-HCl, 100 mM NaCl, 5 mM EDTA, 1% sodium deoxycholate, 4% Tripton X-100, 0.4% SDS), protein of the pig retina and crystalline lens was dissolved. In total, 20 µg of protein was separated through electrophoresis (Bolt 4-12% Bis-Tris Plus Gel, 10-well, Invitrogen, California, US) and then was transferred to PVDF Membrane (Immobilon-P Transfer Membrane Filter, Merck Millipore, Germany). The transferred membrane was reacted with BMP2 and VEGF primary antibody (BMP2: Anti-BMP2 antibody, Abcam, UK, 1:400 VEGF A: VEGF A PolyClonal Antibody, Protein Tech Group, US, 1:100). After treatment with a secondary antibody (Anti-rabbit IgG, HRP-linked Antibody, Cell Signaling, US, 1:2000) and ECL Prime Western Blotting Detection reagent (GE Healthcare, US), densitometry analysis was carried out using a Kodark 4000R (Kodark, New York, NY, US).
To quantify VEGF and BMP2 in the pig vitreous humor, undiluted pig vitreous samples were used with Human VEGF Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, US) for VEGF and Porcine Bone Morphogenetic Protein 2 ELISA Kit (Mybiosource, San Diego, CA, US) for BMP to conduct ELISA in line with the manufacturer's instructions.
Structural abnormalities were observed mostly in the inner plexiform layer. Compared to the control group, decreases in the numbers of nuclei in the ganglion cell layer and the inner nuclear layer were observed among diabetic subjects (Figure 1). In the retina sections produced by slicing the eyeballs horizontally, the area 5.0 mm from the optic nerve was micrographed. When measuring the average numbers of nuclei in the ganglion cell layer and inner nuclear layer within 104 µm2 in two sections per subject, the result on the ganglion cell layer was 3.78±0.60 for diabetic pigs and 5.57±1.07 for control group pigs, showing a statistically significant difference (P<0.05), and 23.11±1.71 for diabetic pigs and 24.54±1.38 for control group pigs on the inner nuclear layer (P=0.17), respectively (Table 1).
When comparing the BMP2 expression in diabetic pig retinas and control group retinas through immunohistochemical staining, the diabetic pig retinas showed an overall increase in expression of BMP2, near the inner segment of photoreceptor and retinal blood vessels (Figure 2).
BMP2 expression in diabetic pig retina and control group retina were compared through Western blotting. The average BMP2/actin level of diabetic pig retina was 1.19±0.05, showing a statistically significant increase compared to the 1.06±0.03 of the control group retina (P=0.016) (Figure 3A). When BMP2 expression in diabetic pig crystalline lenses and control group crystalline lenses were compared, the average BMP2/actin level of diabetic pig crystalline lenses and control group crystalline lenses were 0.72±0.13 and 0.68±0.07, respectively and no difference was found (P=0.730) (Figure 3B).
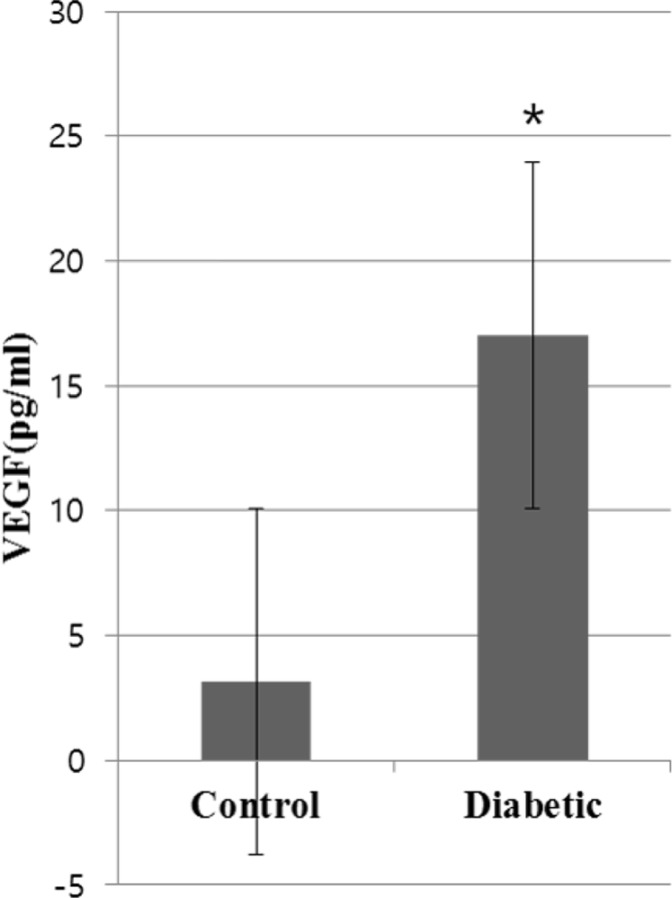
When comparing VEGF expression in the vitreous humor of diabetic subjects and control group pigs through ELISA, the average level in diabetic subjects was 17.02±11.62 (pg/mL), demonstrating an increase compared to that of the control group, at 3.16±3.54 (pg/mL), a statistically significant difference (P<0.05) (Figure 4). BMP2 expression in the vitreous humor of diabetic subjects was not observed in ELISA.
Hussein et al. [6] first reported an increase in BMP2 expression in proliferative diabetic retinopathy using human retina and experimental mice, indicating that BMP2 may be a potential factor in diabetic retinopathy. The experimental subjects of this study were pigs, which are similar to humans compared to most other large-sized mammals. This study aimed to identify changes in the retina and crystalline lens during the early stage of diabetes and to assess the level of BMP2 expression.
Over the past several years, cell-specific antibodies have been used to observe changes in streptozotocin-induced diabetic retinas. The thinning of the ganglion cell layer and the inner layer of the retina exhibit decreased glial activity [28], and significant reduction of cell density [9] has been reported in regard to this. Such activity can change depending on the animal species subjected to the experiment, the method used to develop diabetes, the duration of diabetes, and the type of treatment received. According to Kern et al. [10] the decrease in the number of ganglion cells and the thinning of the inner layer of the retina begin around four weeks to nine months after the development of diabetes. Examination of the histological structure of retinas collected from pigs in their 16th week of diabetes found structural abnormalities in the inner plexiform layer and a decrease in the number of nuclei in the ganglion cell layer.
We observed the increase in BMP2 expression near the inner segment of photoreceptor and blood vessels of the retina. Many other experimental models have shown that BMP2 is secreted from vascular endothelial cells, and the study by Zhang et al. [11] concluded that a high blood glucose level increases the secretion of BMP2 in the endothelial cells of micro vessels.
The crystalline lens of the eyeball can largely be divided into epithelial cells and differentiated fiber cells. One study found that fibroblast growth factor (FGF) and insulin-like growth factor (IGF) are involved in proliferation of epithelial cells and differentiation of fiber cells in the crystalline lens. However, in addition to these two growth factors, a study by Belecky TL et al. used the BMP antibody to show that the BMP signaling pathway is also involved in differentiation of fiber cells in the crystalline lens during development [12]. Moreover, Boswell BA et al. [13] stated that BMP2 plays an essential role in the FGF-induced secondary differentiation of fiber cells in the crystalline lens. Diabetes is known to cause cataract due to structural changes derived from the progressive glycation of the crystalline lens and the deposition of high-molecular substances in STZ-induced diabetic mice [14]; this is mainly because diabetes-induced oxidative stress occurs through the polyol pathway in the crystalline lens [15]. We found in this study that BMP2 expression slightly increased in the crystalline lens of diabetic subjects compared to that of the control group, however the difference was not statistically significant. As the BMP signaling pathway is involved in the reaction to oxidative stress, inflammation, and blood vessel formation [5], BMP2 may be involved in the development of diabetes-induced cataracts; additional study on this is necessary.
Hussein et al. [6] reported an increase in BMP2 expression in the vitreous humor of humans suffering from proliferative diabetic retinopathy. In the present study, BMP2 expression was not identified through Western blotting in vitreous. BMP is known to be secreted to the vitreous humor after being produced in the retina in the initial stage of development in the chicken embryo [12] and is perceived as the source of BMP2 secretion [1617]. Thus, it may have not been identified here as pigs in their 16th week of diabetes were the subjects of this study.
VEGF is widely known to be a mediator of vascularization and inflammation in diabetic retinopathy. Akeel et al. [5] reported that BMP2 increases inflammatory mediators such as VEGF and IL-6 in the osteoblasts of humans and causes vascularization. Moreover, a previous study showed that, when cultivated Müller cells were treated with BMP2, the concentration level of VEGF increased compared to the group that was not treated with BMP2. [6] Nevertheless, the reaction pathway between BMP2 and VEGF has not been clearly identified, suggesting further studies are necessary.
There are limitations to this study. First, the small number of subject pigs may limit the statistical power to identify differences in the numbers of nuclei in the ganglion cell layer and the inner nuclear layer of diabetic retinas and control group retinas. Second, possible direct effects on the eye of Streptozotocin chemical which was intravenously injected to induce diabetes cannot be totally excluded.
In conclusion, structural abnormalities in the inner plexiform layer of streptozotocin-induced diabetic pigs, decreases in the numbers of nuclei in the ganglion cell layer, and an increase in BMP2 expression in the retina of diabetic subjects were identified compared to the control group. BMP2 may be a mediator of diabetic retinopathy and further research is needed.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2016R1A1A1A05005484) and by a grant from the Next-Generation BioGreen 21 Program (Project No. PJ01109301) funded by Rural Development Administration, Republic of Korea.
Notes
References
1. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35(3):556–564. PMID: 22301125.

2. van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Abràmoff MD. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010; 51(7):3660–3665. PMID: 20130282.

3. Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, Garmager A, Wit F, Kucukevcilioglu M, van Velthoven ME, DeVries JH, Mullins RF, Kuehn MH, Schlingemann RO, Sonka M, Verbraak FD, Abràmoff MD. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016; 113(19):E2655–E2664. PMID: 27114552.

4. Guo J, Wu G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012; 23(1-2):61–67. PMID: 22421241.

5. Akeel S, El-Awady A, Hussein K, El-Refaey M, Elsalanty M, Sharawy M, Al-Shabrawey M. Recombinant bone morphogenetic protein-2 induces up-regulation of vascular endothelial growth factor and interleukin 6 in human pre-osteoblasts: role of reactive oxygen species. Arch Oral Biol. 2012; 57(2):445–452. PMID: 22041018.

6. Hussein KA, Choksi K, Akeel S, Ahmad S, Megyerdi S, El-Sherbiny M, Nawaz M, Abu El-Asrar A, Al-Shabrawey M. Bone morphogenetic protein 2: a potential new player in the pathogenesis of diabetic retinopathy. Exp Eye Res. 2014; 125:79–88. PMID: 24910902.

7. Lee MS, Song KD, Yang HJ, Solis CD, Kim SH, Lee WK. Development of a type II diabetic mellitus animal model using Micropig®. Lab Anim Res. 2012; 28(3):205–208. PMID: 23091521.

8. van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Verbraak FD, Abràmoff MD. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009; 50(7):3404–3409. PMID: 19151397.

9. Park HS, Park SJ, Park SH, Chun MH, Oh SJ. Shifting of parvalbumin expression in the rat retina in experimentally induced diabetes. Acta Neuropathol. 2008; 115(2):241–248. PMID: 17989985.

10. Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008; 586(18):4401–4408. PMID: 18565995.

11. Zhang M, Zhou SH, Zhao S, Li XP, Liu LP, Shen XQ. Pioglitazone can downregulate bone morphogenetic protein-2 expression induced by high glucose in human umbilical vein endothelial cells. Pharmacology. 2008; 81(4):312–316. PMID: 18311072.

12. Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002; 129(16):3795–3802. PMID: 12135918.

13. Boswell BA, Overbeek PA, Musil LS. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008; 324(2):202–212. PMID: 18848538.

14. Perry RE, Swamy MS, Abraham EC. Progressive changes in lens crystallin glycation and high-molecular-weight aggregate formation leading to cataract development in streptozotocin-diabetic rats. Exp Eye Res. 1987; 44(2):269–282. PMID: 3582512.

15. Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003; 14:S233–S236. PMID: 12874437.

16. Hussein KA, Zakhary IE, Elawady AR, Emam HA, Sharawy M, Baban B, Akeel S, Al-Shabrawey M, Elsalanty ME. Difference in soft tissue response between immediate and delayed delivery suggests a new mechanism for recombinant human bone morphogenetic protein 2 action in large segmental bone defects. Tissue Eng Part A. 2012; 18(5-6):665–675. PMID: 21981405.

17. Mathura JR Jr, Jafari N, Chang JT, Hackett SF, Wahlin KJ, Della NG, Okamoto N, Zack DJ, Campochiaro PA. Bone morphogenetic proteins-2 and -4: negative growth regulators in adult retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2000; 41(2):592–600. PMID: 10670493.
Figure 1
Hematoxylin and Eosin staining of diabetic pig retina. Gross structural abnormalities were observed, particularly in the inner plexiform layer, compared to the normal retina. There are decreases in the numbers of nuclei in the ganglion cell layer and inner nuclear layer in the retina of diabetic subjects.
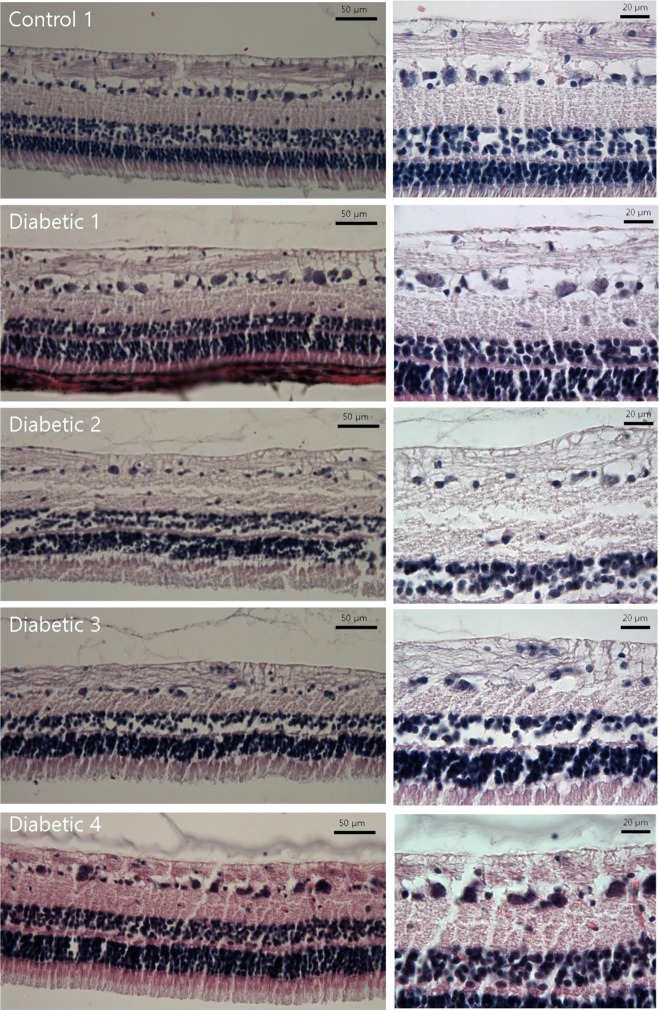
Figure 2
BMP2 expression in diabetic pig retinas. Immunohistochemistry of BMP2 in normal (left) and diabetic pig retinas (right). Increase in the expression of BMP2 in diabetic subjects is observed (Green stain) compared to the normal retina. BMP2 is increased especially in the photoreceptor inner segment (Arrowhead) and in relation to retinal vessels (Arrow). DAPI, 4',6'-diamidino-2-phenylindole; FITC, Fluorescein isothiocyanate conjugated; IL B4, Isolectin B4; GSL 1, Griffonia Simplicifolia Lectin I; NFL, Nerve fiber layer; GCL, Ganglion cell layer; IPL, Inner plexiform layer; INL, Inner nuclear layer; OPL, Outer plexiform layer; ONL, Outer nuclear layer; IS, Inner segments; OS, Outer segments.
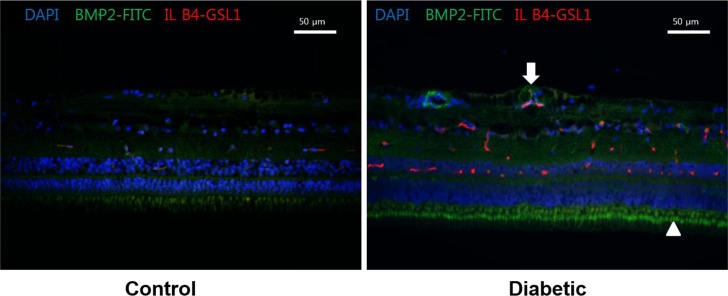
Figure 3
(A) BMP2 expression in diabetic pig retina. Western blot analysis of BMP2 in normal and diabetic pigs showing higher abundance of BMP2 in the retina of diabetic pigs compared to the control group. (B) BMP2 expression in diabetic pig crystalline lenses. Western blot analysis of BMP2 in normal and diabetic pigs showing similar abundance of BMP2 in the crystalline lens of diabetic pigs compared to the control group.
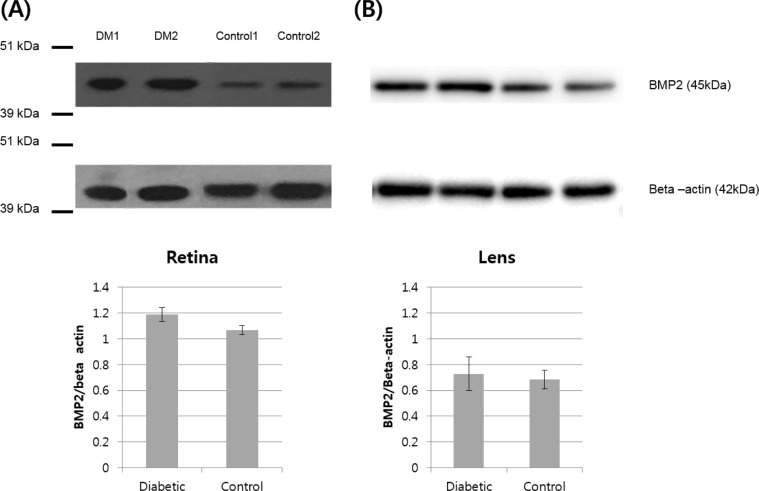
Figure 4
VEGF expression in diabetic pig vitreous. Enzyme-linked immunosorbent assay (ELISA) of VEGF in normal and diabetic pigs showing upregulation of VEGF in the vitreous of diabetic pigs compared to the control group (*P value <0.05 versus control group).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download