Abstract
Korl:ICR mice, established by the Korean National Institute of Food and Drug Safety Evaluation (NIFDS), are characterized based on their genetic variation, response to gastric injury, and response to constipation inducers. To compare the inhibitory responses of ICR stocks obtained from three different sources to the anticancer drug cisplatin (Cis), alterations in tumor volume, histopathological structure, and toxicity were examined in Sarcoma 180 tumor-bearing Korl:ICR, A:ICR (USA source), and B:ICR (Japan source) mice treated with low and high concentrations of Cis (L-Cis and H-Cis, respectively). Tumor size and volume were lower in H-Cis-treated mice than in L-Cis-treated mice in all three ICR stocks with no significant differences among stocks. There was a significant enhancement of the necrotizing areas in the histological structures in the L-Cis- and H-Cis-treated groups relative to that in the untreated group. The necrotizing area changes were similar in the Sarcoma 180 tumor-bearing Korl:ICR, A:ICR, and B:ICR mice. However, there were stock-bases differences in the serum biomarkers for liver and kidney toxic effects. In particular, the levels of AST, ALT and BUN increased differently in the three H-Cis-treated ICR stocks, whereas the levels of ALP and CRE were constant. Taken together, the results of the present study indicate that Korl:ICR, A:ICR, and B:ICR mice have similar overall inhibitory responses following Cis treatment of Sarcoma 180-derived solid tumors, although there were some differences in the magnitude of the toxic effects in the three ICR stocks.
ICR mice are one of the most popular strains of outbred mice used in studies on pharmacology, toxicology, oncology, and pharmaceutical product safety testing [12345]. These mice were originally developed by the Institute of Cancer Research in 1947 via the crossing of two males with seven females, known as “Swiss” mice. They were subsequently merged with “Fox Chase” mice in 1948 and began to be commercially produced by several commercial breeding companies in 1959 [136].
Many ICR stocks have been established after long periods of breeding and rearing by several mouse breeders around the world. Among these stocks, Korl:ICR mice were established as an ICR stock by the National Institute of Food and Drug Safety Evaluation (NIFDS) in Korea with its stock name based on the Guidelines for Nomenclature of Mouse and Rat Strains [7]. Recent studies have described the genetic variation, response to gastric injury, and response to constipation inducer characteristics of Kor1:ICR mice. In a genome-wide single nucleotide polymorphism (SNP) analysis, unique genetic variation was identified in Korl:ICR mice when compared with two ICR stocks derived from different sources [8]. However, Korl:ICR mice showed similar overall responses to gastric ulcers induced by EtOH/HCl administration compared to those in A:ICR and B:ICR mice stocks derived from different sources [9]. Also, similar responses to loperamide (Lop)-induced constipation in the transverse colon were detected in three ICR stocks (Korl:ICR, A:ICR, and B:ICR) derived from three difference sources [10]. To date, the inhibitory response to antitumor drugs has not been characterized in Korl:ICR mice.
The present study compared the inhibitory response of three stocks of ICR mice (Korl:ICR, A:ICR, and B:ICR) obtained from difference sources to the anticancer drug cisplatin (Cis). Our results provide the first scientific evidence of very similar inhibitory responses to Cis treatment in Sarcoma 180-induced tumors in Korl:ICR, A:ICR and B:ICR mice, although there were slight differences in the magnitude of the toxic effects of Cis treatment among the ICR stocks.
The mouse Sarcoma 180 cell line was obtained from the Korean Cell Line Bank (Seoul, Korea) and grown in a humidified incubator at 37℃ under 5% CO2 and 95% air in Dulbecco's modified Eagle's medium (DMEM, Thermo Scientific, Waltham, MA, USA) containing 10% fetal bovine serum, 2mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin. Sarcoma 180 cells were maintained in an ascetic form by serial transplantation every 13 days in Korl:ICR mice.
Protocols for the animal experiments were carefully reviewed for ethical and scientific care procedures and approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval PNU-2015-0916). Six-week-old male ICR mice were obtained from three difference sources. Korl:ICR mice were kindly provided by the Department of Laboratory Animal Resources in the National Institute of Food and Drug Safety Evaluation (NIFDS, Cheongju, Korea). The other two stocks of ICR mice were provided from vendors located in the United States (A:ICR) and Japan (B:ICR). All mice were provided with ad libitum access to water and a standard irradiated chow diet (Samtako, Osan, Korea) consisting of moisture (12.5%), crude protein (25.43%), crude fat (6.06%), crude fiber (3.9%), crude ash (5.31%), calcium (1.14%), and phosphorus (0.99%). During the experiment, mice were maintained in a specific pathogen-free (SPF) state under a strict light cycle (lights on at 08:00 A.M. and off at 08:00 P.M.) at 23℃±2℃ and 50%±10% relative humidity. The mice were housed in the Pusan National University-Laboratory Animal Resources Center, which is accredited by the Korea Ministry of Food and Drug Safety (MFDS; Accredited Unit 000231) and AAALAC International (Accredited Unit 001525).
Antitumor activity of Cis in the three ICR stocks was evaluated by using methods described in previous studies [1112], with some modifications. Briefly, Sarcoma 180 cells (5×106 cells) collected from ascetic tumors in Krol:ICR mice were subcutaneously injected into the groin region of six-week-old Korl:ICR, A:ICR, and B:ICR mice (n=28/group; Figure 1A). Subsequently, Sarcoma 180 tumor cell-bearing ICR mice in each stock were randomly divided into one of four groups. The first group (No group, n=7) did not receive any treatment, the second group (Vehicle-treated group, n=7) received a constant volume of 1x PBS every 2 days for 6 days. The other two groups were intraperitoneally received either a low concentration of Cis (1.5 mg/kg; L-Cis-treated group, n=7) or a high concentration of Cis (3 mg/kg; H-Cistreated group, n=7) every 2 days for 6 days. At 24 h after final treatment, all animals in each group were euthanized by placing them in a chamber filled with CO2 gas, after which tumor and blood samples were collected (Figure 1B).
The weights of tumors dissected from Sarcoma 180 tumor-bearing ICR mice were determined by using an electronic balance (Mettler Toledo, Greifensee, Switzerland) according to the Korean MFDS guideline. After measuring tumor length and width by using calipers (Matusutoyo, Tokyo, Japan), tumor volume was calculated using following formula: Tumor volume=AB2/2, where A is the length of tumor, and B is the width of tumor.
Tumor tissues were excised from Sarcoma 180 tumor-bearing ICR mice, fixed in 10% formalin, embedded in paraffin wax, routinely processed, and then sectioned into 4 µm thick slices. The tumor sections were then stained with hematoxylin and eosin (H&E), after which they were examined by light microscopy (Leica Microsystems, Wetzlar, Germany) to detect alterations in histological structure.
After fasting for 8 h prior to euthanasia, whole blood from each mouse in all groups was collected from their abdominal veins and incubated for 30 min at room temperature in a serum separating tube (BD Container, Franklin Lakes, NJ, USA). Serum was obtained by centrifugation at 1,500×g and analyzed to determine alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), and creatinine (CRE) levels by using an Automatic Biochemical Analyzer (BS-120, Mindray, China). All assays were conducted in duplicate using fresh serum.
One-way ANOVA (SPSS for Windows, release 10.10, standard version; IBM, Chicago, IL, USA) was used to identify significant differences among No, Vehicle-treated, and Cis-treated groups as well as among Korl:ICR, A:ICR, and B:ICR. All values are reported as means±standard deviations (SD) and a P value of <0.05 was considered significant.
To compare the antitumor activity responses to Cis treatment in ICR mice stocks obtained from three different sources, alterations in tumor size and volume were measured in Sarcoma 180 tumor-bearing Korl:ICR, A:ICR, and B:ICR mice treated with low or high concentrations of Cis. After 6 days of Cis treatment, skin tumor size gradually decreased in both L-Cis- and H-Cis-treated groups. The size decrease was greater in the L-Cis and H-Cis-treated groups than that in the Vehicle-treated group. Moreover, the size decreases were similar in the three ICR stocks (Figure 2A). Furthermore, tumor volumes collected from Sarcoma 180 tumor-bearing ICR mice were smaller in the L-Cis- and H-Cis-treated groups than in the Vehicle-treated group. In both L-Cisand H-Cis-treated groups of Korl:ICR, A:ICR, and B:ICR mice, the decreases in tumor volume were similar, although the greatest decreases were observed in the HCis- treated group (Figure 2B). Therefore, the results indicate that Korl:ICR, A:ICR, and B:ICR exhibit similar patterns of antitumor activity to Cis treatment in Sarcoma 180 cells-induced tumors.
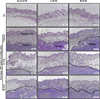
To examine whether the antitumor activity of Cis is accompanied by histopathological alterations in the tumor tissue of ICR stocks from three different sources, alterations in the histological structure of tumor were observed in Korl:ICR, A:ICR, and B:ICR mice injected with Sarcoma 180 cells. In the Vehicle-treated group, solid tumors were well formed in the area of intradermal fat and dermis compared with the No group. Any significant difference was not observed in tumor formation of Korl:ICR, A:ICR, and B:ICR mice after the injection of Sarcoma 180 cells (Figure 3). In the L-Cis- and H-Cis-treated groups, the necrotizing area and the number of dead cells were significantly greater than those in the Vehicle-treated group. These patterns on the tumor tissue were constantly observed in three stocks of ICR mice from different sources, although there are non-significant difference between L-Cis- and H-Cis-treated group. (Figure 3). The above results suggest that similar alterations in tumor histopathological structure were induced by Cis treatment in the three stocks of ICR mice.
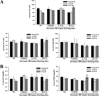
Finally, we compared the toxic responses in the liver and kidney of the three ICR mice stocks to Cis treatment. Alterations in the level of serum biomarkers indicating liver and kidney toxicity were measured in the blood serum of Sarcoma 180 tumor-bearing Korl:ICR, A:ICR, and B:ICR mice treated with Cis. Significant differences in toxic responses were detected in two liver toxicity markers and one kidney toxicity marker. In the H-Cistreated group, a significant difference on the level of AST and ALT were detected among three ICR stocks, while the level of ALP in the three ICR stocks were maintained at a consistent level (Figure 4A). In contrast, the BUN level varied among the three ICR stocks in the L-Cis- and H-Cis-treated groups. Specifically, high BUN levels were detected in B:ICR mice treated with both L-Cis and H-Cis and in Korl:ICR mice treated with H-Cis compared with A:ICR mice (Figure 4B). Overall, the above results indicate that there are few difference on the toxic responses to the antitumor drug Cis in Sarcoma 180 tumor-bearing ICR stocks from three different sources.
Cancer animal models have been widely used to evaluate the efficacy of therapeutic drugs, to investigate pathophysiological characters of disease, to examine the molecular mechanisms associated with therapeutic drugs, to identify novel targets and biomarkers of drugs [13], and to establish pharmacodynamic/pharmacokinetic relationships [14,15]. In particular, Sarcoma 180 tumor-bearing ICR mice have been used to evaluate the antitumor activity of various natural products and chemical compounds [16]. However, differences among ICR stocks derived from different sources and the effect of those differences on the inhibitory activity of anticancer drugs have not been reported. In this study, we used two different concentrations (1.5 and 3 mg/kg) of Cis to inhibit Sarcoma 180 induced tumors in ICR mice, and differences in the inhibitory responses to Cis treatment in Sarcoma 180 tumor-bearing ICR stocks derived from three different sources were investigated. The results presented in this report provide the first evidence that Korl:ICR, A:ICR, and B:ICR mice show similar responses to tumor development after the injection of Sarcoma 180 cells and that Cis has similar antitumor activity among the three ICR stocks, although additional research is needed to quantify stock-based effects fully.
ICR mice, as an outbred stock, have become the most commonly used mice in research after first being established by Hauschka at the Institute for Cancer Research [6]. Especially, they have commonly been used to produce animal models for tumor-based studies and to evaluate the therapeutic effects of antitumor drugs. Antitumor activity against Sarcoma 180 induced solid tumors has been successfully detected in ICR mice after treatment with cauliflower mushroom [16], 4-O-methylgluccuronoxylan [17], and coumarin [18]. However, the mice used in each of those studies were derived from only one company or institute with no comparison of inhibitory effects between ICR stocks derived from different sources. Therefore, this study was undertaken to characterize the response of three ICR stocks to tumor development induced by Sarcoma 180 cells and to compare the antitumor effects of Cis in Sarcoma 180 tumor-bearing ICR mice from the three sources. The results indicated that the antitumor response of Korl:ICR mice to Cis treatment of tumors induced by Sarcoma 180 cells was very similar to the responses in two other commercially available stocks of ICR mice.
Many studies have reported difference in strain-specific susceptibility of mice to development, metastasis, and regression of tumors, including Sarcoma 180 cell tumors. Various growth patterns have been detected in different inbred mouse strains after injection of Sarcoma 180 cells [1920]. Strain-based differences in the regression of Sarcoma 180 cells were detected in ICR mice and DBA/ 2 mice [21]. Alterations in tumor incidence were investigated in several strains of mice including ICR/Ha, ICR/albino, C58, and C3H/HeNIer [22]. The development of pulmonary metastasis was assayed in multiple inbred mouse strains to identify the influence of host genetic variation during metastasis [23]. However, stock-based differences in ICR mice to antitumor drugs have not been investigated in mice with Sarcoma 180 derived tumors, though stock differences among ICR mice have arisen in newly established stocks (founder effects) in different locations worldwide and in stocks that have been bred for a long time (drift effects) [8]. The present study provides the first evidence of stock-based differences in Cis treatment responses in Sarcoma 180 tumor-bearing ICR mice derived from three different sources.
Taken together, we investigated the antitumor responses to Cis treatment (two concentrations; 1.5 and 3 mg/kg) in Sarcoma 180 tumor-bearing ICR mice from three different sources. Following Cis treatment, the three ICR stocks (Korl:ICR, A:ICR, and B:ICR mice) had similar overall tumor sizes and volumes, and histopathological structures, but some significant differences in biomarker levels for liver and kidney toxicity were observed. Our results suggest that Korl:ICR mice can be used as widely as other ICR mice provided from commercial vendors in the evaluation of the therapeutic effects of antitumor drugs.
Figures and Tables
Figure 1
Schematic diagram for the production of Sarcoma 180 tumor-bearing ICR mice and experimental schedule for Cis treatment.
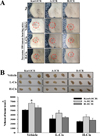
Figure 2
Tumor size and volume. (A) Morphological features of tumor observed on the skin surface of ICR mice derived from three different sources. Dashed circle indicates tumor area. (B) Tumor volume from Sarcoma 180 tumor-bearing ICR mice. Tumor volume was calculated using the formula described in the materials and methods. The data are presented as a mean±standard deviation (SD) of three replicates. *, P<0.05 compared to Korl:ICR mice.
Acknowledgments
We thank Jin Hyang Hwang, the animal technician, for directing the animals care at the Laboratory Animal Resources Center. This project was supported by a grant of BIOREIN (Laboratory Animal Bio Resources Initiative) from the Ministry of Food and Drug Safety in 2015.
References
1. Rice MC, O'Brien SJ. Genetic variance of laboratory outbred Swiss mice. Nature. 1980; 283(5743):157–161.
2. Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993; 27(2):116–123.
3. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005; 37(11):1181–1186.
4. O'Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009; 14(5):511–522.
5. Zhong SZ, Ge QH, Qu R, Li Q, Ma SP. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J Neurol Sci. 2009; 277(1-2):58–64.
6. Lynch CJ. The so-called Swiss mouse. Lab Anim Care. 1969; 19(2):214–220.
7. Snell GD. Biology of the Laboratory Mouse. 1st ed. New York: McGraw-Hill;1941. p. 1–497.
8. Hwang SJ, Cho YM, Shin HJ, Kim HD, Choi KM, Kwon KC, Lee SH, Shin HJ, Shin HD, Chung MW. Stratification of outbred ICR mice stocks by genetic variation. Acad J Biotechnol. 2016; 4(11):365–371.
9. Song SH, Kim JE, Go J, Koh EK, Sung JE, Lee HA, Choi KM, Kim HD, Jung YS, Kim KS, Hwang DY. Comparison of the response using ICR mice derived from three different sources to ethanol/hydrochloric acid-induced gastric injury. Lab Anim Res. 2016; 32(1):56–64.
10. Kim JE, Yun WB, Sung JE, Lee HA, Choi JY, Choi YS, Jung YS, Kim KS, Hwang DY. Characterization the response of Korl:ICR mice to loperamide induced constipation. Lab Anim Res. 2016; 32(4):231–240.
11. Wu HT, Lu FH, Su YC, Ou HY, Hung HC, Wu JS, Yang YC, Chang CJ. In vivo and in vitro anti-tumor effects of fungal extracts. Molecules. 2014; 19(2):2546–2556.
12. da Silva, Santos NA, Carvalho Rodrigues MA, Rodrigues JL, Barbosa Junior F, Santos AC. Effect of diabetes on biodistribution, nephrotoxicity and antitumor activity of cisplatin in mice. Chem Biol Interact. 2015; 229:119–131.
13. Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov. 2003; 2(7):566–580.
14. Bueters T, Ploeger BA, Visser SA. The virtue of translational PKPD modeling in drug discovery: selecting the right clinical candidate while sparing animal lives. Drug Discov Today. 2013; 18(17-18):853–862.
15. Fan J, de Lannoy IA. Pharmacokinetics. Biochem Pharmacol. 2014; 87(1):93–120.
16. Kimura T. Natural products and biological activity of the pharmacologically active cauliflower mushroom Sparassis crispa. Biomed Res Int. 2013; 2013:982317.
17. Hashi M, Takeshita T. The host-mediated anti-tumor effect of 4-O-methylgluccuronoxylan. Agric Biol Chem. 1979; 43(5):961–967.
18. Stefanova TH, Nikolova NJ, Toshkova RA, Neychev HO. Antitumor and immunomodulatory effect of coumarin and 7-hydroxycoumarin against Sarcoma 180 in mice. J Exp Ther Oncol. 2007; 6(2):107–115.
19. Snell GD, Russell E, Fekete E, Smith P. Resistance of various inbred strains of mice to tumor homoiotransplants, and its relation to the H-2 allele which each carries. J Natl Cancer Inst. 1953; 14(3):485–491.
20. Strong LC. Indications of tissue specificity in a transplantable sarcoma. J Exptl Med. 1924; 39(3):447–456.
21. Bradner WT, Pindell MH. Strain specificity of stimulated regression of sarcoma 180. Cancer Res. 1965; 25(6):859–864.
22. Diller IC, Donnelly AJ, Fisher ME. Isolation of pleomorphic, acid-fast organisms from serveral strains of mice. Cancer Res. 1967; 27(8):1402–1408.
23. Vikis HG, Jackson EN, Krupnick AS, Franklin A, Gelman AE, Chen Q, Piwnica-Worms D, You M. Strain-specific susceptibility for pulmonary metastasis of sarcoma 180 cells in inbred mice. Cancer Res. 2010; 70(12):4859–4867.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download