1. Ozgediz D, Chu K, Ford N, Dubowitz G, Bedada AG, Azzie G, Gerstle JT, Riviello R. Surgery in global health delivery. Mt Sinai J Med. 2011; 78(3):327–341. PMID:
21598260.

2. Mock C, Cherian MN. The global burden of musculoskeletal injuries: challenges and solutions. Clin Orthop Relat Res. 2008; 466(10):2306–2316. PMID:
18679760.

3. Bandela V, Munagapati B, Karnati RK, Venkata GR, Nidudhur SR. Osteoporosis: Its Prosthodontic Considerations-A Review. J Clin Diagn Res. 2015; 9(12):ZE01–ZE04.
4. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006; 17(12):1726–1733. PMID:
16983459.

5. Ray NF, Chan JK, Thamer M, Melton LJ 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997; 12(1):24–35. PMID:
9240722.

6. Tajeu GS, Delzell E, Smith W, Arora T, Curtis JR, Saag KG, Morrisey MA, Yun H, Kilgore ML. Death, debility, and destitution following hip fracture. J Gerontol A Biol Sci Med Sci. 2014; 69(3):346–353. PMID:
23873945.

7. Pothiwala P, Evans EM, Chapman-Novakofski KM. Ethnic variation in risk for osteoporosis among women: a review of biological and behavioral factors. J Womens Health (Larchmt). 2006; 15(6):709–719. PMID:
16910903.

8. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000; 21(2):115–137. PMID:
10782361.

9. Ralston SH. Analysis of gene expression in human bone biopsies by polymerase chain reaction: evidence for enhanced cytokine expression in postmenopausal osteoporosis. J Bone Miner Res. 1994; 9(6):883–890. PMID:
8079663.

10. Kinoshita T, Kobayashi S, Ebara S, Yoshimura Y, Horiuchi H, Tsutsumimoto T, Wakabayashi S, Takaoka K. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone. 2000; 27(6):811–817. PMID:
11113392.

11. Ahlström M, Lamberg-Allardt C. Rapid protein kinase A--mediated activation of cyclic AMP-phosphodiesterase by parathyroid hormone in UMR-106 osteoblast-like cells. J Bone Miner Res. 1997; 12(2):172–178. PMID:
9041048.

12. Civitelli R, Bacskai BJ, Mahaut-Smith MP, Adams SR, Avioli LV, Tsien RY. Single-cell analysis of cyclic AMP response to parathyroid hormone in osteoblastic cells. J Bone Miner Res. 1994; 9(9):1407–1417. PMID:
7817824.

13. Wronski TJ, Yen CF, Qi H, Dann LM. Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology. 1993; 132(2):823–831. PMID:
8425497.

14. Degerman E, Smith CJ, Tornqvist H, Vasta V, Belfrage P, Manganiello VC. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci U S A. 1990; 87(2):533–537. PMID:
2153956.

15. Marchmont RJ, Ayad SR, Houslay MD. Purification and properties of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981; 195(3):645–652. PMID:
6274308.

16. Bayat M, Amini A, Rezaie F, Bayat S. Patents of Pentoxifylline Administration on some diseases and chronic wounds. Recent Pat Regen Med. 2014; 4(2):137–143.

17. Vashghani Farahani MM, Masteri Farahani R, Mostafavinia A, Abbasian MR, Pouriran R, Noruzian M, Ghoreishi SK, Aryan A, Bayat M. Effect of Pentoxifylline Administration on an Experimental Rat Model of Femur Fracture Healing With Intramedullary Fixation. Iran Red Crescent Med J. 2015; 17(12):e29513. PMID:
26756019.

18. Çakmak G, Þahin MÞ, Özdemİ BH, Karadenİ E. Effect of pentoxifylline on healing of segmental bone defects and angiogenesis. Acta Orthop Traumatol Turc. 2015; 49(6):676–682. PMID:
26511696.
19. Atalay Y, Gunes N, Guner MD, Akpolat V, Celik MS, Guner R. Pentoxifylline and electromagnetic field improved bone fracture healing in rats. Drug Des Devel Ther. 2015; 9:5195–5201.

20. Bohn JC, Schussel JL, Stramandinoli-Zanicotti RT, Sassi LM. Tissue repair in osteoradionecrosis using pentoxifylline and tocopherol--report of three cases. Oral Maxillofac Surg. 2016; 20(1):97–101. PMID:
26251132.

21. Erken HY, Burc H, Aydogan M. The Effect of Pentoxifylline on Spinal Fusion: An Experimental Study in Rabbits. Spine (Phila Pa 1976). 2014; 39(11):27.
22. Labib GS, Farid RM. Osteogenic effect of locally applied Pentoxyfilline gel:
in vitro and
in vivo evaluations. Drug Deliv. 2015; 22(8):1094–1102. PMID:
24555662.
23. Queiroz-Junior CM, Bessoni RL, Costa VV, Souza DG, Teixeira MM, Silva TA. Preventive and therapeutic anti-TNF-α therapy with pentoxifylline decreases arthritis and the associated periodontal co-morbidity in mice. Life Sci. 2013; 93(9-11):423–428. PMID:
23911669.

24. Aydin K, Sahin V, Gürsu S, Mercan AS, Demir B, Yildirim T. Effect of pentoxifylline on fracture healing: an experimental study. Eklem Hastalik Cerrahisi. 2011; 22(3):160–165. PMID:
22085352.
25. Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. Eur J Pain. 2009; 13(3):253–262. PMID:
18554967.

26. Horiuchi H, Saito N, Kinoshita T, Wakabayashi S, Tsutsumimoto T, Otsuru S, Takaoka K. Enhancement of recombinant human bone morphogenetic protein-2 (rhBMP-2)-induced new bone formation by concurrent treatment with parathyroid hormone and a phosphodiesterase inhibitor, pentoxifylline. J Bone Miner Metab. 2004; 22(4):329–334. PMID:
15221490.

27. Beþe NS, Ozgüroğ M, Kamberoğ K, Karahasanoglu T, Ober A. Pentoxifylline in the treatment of radiation-related pelvic insufficiency fractures of bone. Radiat Med. 2003; 21(5):223–227. PMID:
14632299.
28. Robin JC, Ambrus JL. Study of antiosteoporotic agents in tissue culture. J Med. 1984; 15(4):319–322. PMID:
6098626.
29. Robin JC, Ambrus JL. Studies on osteoporoses. XI. Effects of a methylxanthine derivative. A preliminary report. J Med. 1983; 14(2):137–145. PMID:
6310016.
30. Magremanne M, Reychler H. Pentoxifylline and tocopherol in the treatment of yearly zoledronic acid-related osteonecrosis of the jaw in a corticosteroid-induced osteoporosis. J Oral Maxillofac Surg. 2014; 72(2):334–337. PMID:
23891014.

31. Takami M, Cho ES, Lee SY, Kamijo R, Yim M. Phosphodiesterase inhibitors stimulate osteoclast formation via TRANCE/RANKL expression in osteoblasts: possible involvement of ERK and p38 MAPK pathways. FEBS Lett. 2005; 579(3):832–838. PMID:
15670856.

32. Hosny KM. Alendronate sodium as enteric coated solid lipid nanoparticles; preparation, optimization, and
in vivo evaluation to enhance its oral bioavailability. PLoS One. 2016; 11(5):e0154926. PMID:
27148747.
33. Imai K. Alendronate sodium hydrate (oral jelly) for the treatment of osteoporosis: review of a novel, easy to swallow formulation. Clin Interv Aging. 2013; 8:681–688. PMID:
23766643.

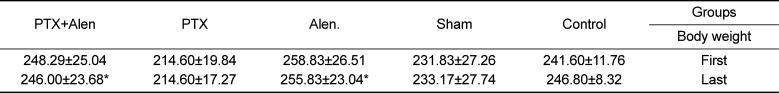
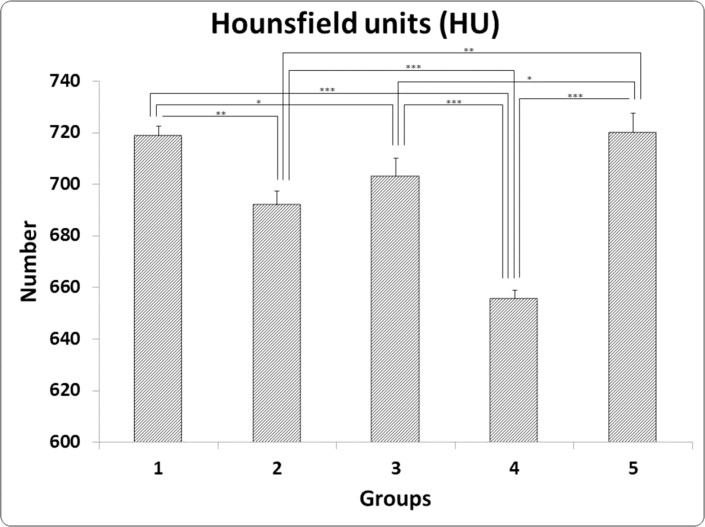
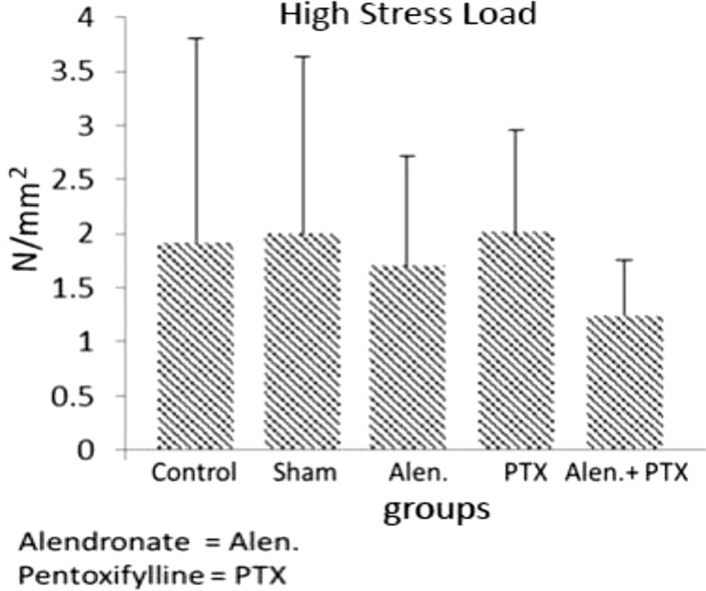
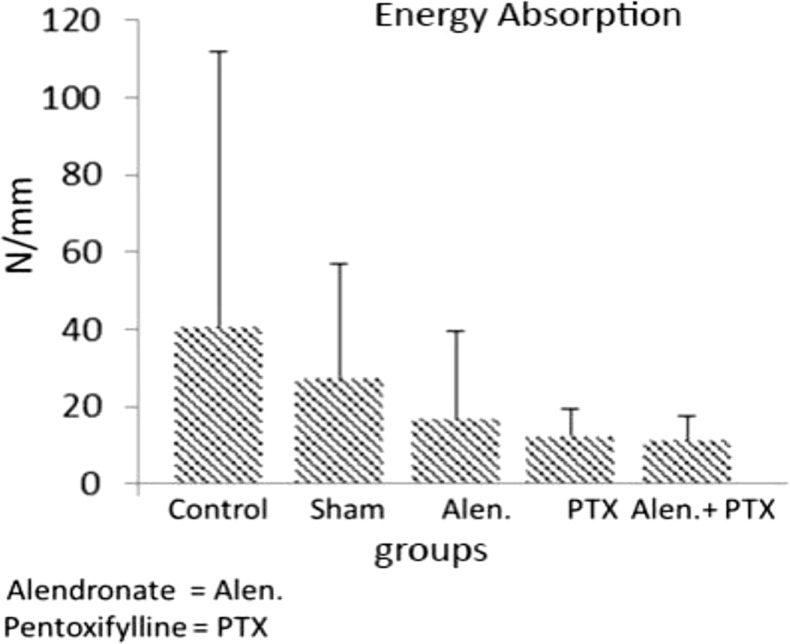
34. Mostafavinia A, Ahadi R, Abdollahifar MA, Ghorishi SK, Jalalifirouzkouhi A, Bayat M. Evaluation of the Effects of Low-Level Laser Therapy on Biomechanical Properties and Hounsfield Unit of Partial Osteotomy Healing in an Experimental Rat Model of Type I Diabetes and Osteoporosis. under publication. DOI:
10.1089/pho.2016.4191.
35. Lee SW, Jeon TJ, Biswal S. Fracture Healing Effects of Locally-Administered Adipose Tissue-Derived Cells. Yonsei Med J. 2015; 56(4):1106–1113. PMID:
26069136.

36. Little DG, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Joint Surg Br. 2007; 89(4):425–433. PMID:
17463107.

37. Caro AC, Tucker JJ, Yannascoli SM, Dunkman AA, Thomas SJ, Soslowsky LJ. Efficacy of various analgesics on shoulder function and rotator cuff tendon-to-bone healing in a rat (Rattus norvegicus) model. J Am Assoc Lab Anim Sci. 2014; 53(2):185–192. PMID:
24602546.
38. Freidouni M, Nejati H, Salimi M, Bayat M, Amini A, Noruzian M, Asgharie MA, Rezaian M. Evaluating glucocorticoid administration on biomechanical properties of rats' tibial diaphysis. Iran Red Crescent Med J. 2015; 17(3):e19389. PMID:
26019900.

39. Renno AC, de Moura FM, dos Santos NS, Tirico RP, Bossini PS, Parizotto NA. Effects of 830-nm laser, used in two doses, on biomechanical properties of osteopenic rat femora. Photomed Laser Surg. 2006; 24(2):202–206. PMID:
16706700.

40. Ibrahim N, Mohamed N, Shuid AN. Update on statins: hope for osteoporotic fracture healing treatment. Curr Drug Targets. 2013; 14(13):1524–1532. PMID:
23876090.

41. Lewiecki EM. New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol. 2011; 7(11):631–638. PMID:
21931340.

42. Bayat M, Chelcheraghi F, Piryaei A, Rakhshan M, Mohseniefar Z, Rezaie F, Bayat M, Shemshadi H, Sadeghi Y. The effect of 30-day pretreatment with pentoxifylline on the survival of a random skin flap in the rat: an ultrastructural and biomechanical evaluation. Med Sci Monit. 2006; 12(6):BR201–BR207. PMID:
16733477.
43. Velaei K, Bayat M, Torkman G, Rezaie F, Amini A, Noruzian M, Tavassol A, Bayat M. Evaluating the effects of pentoxifylline administration on experimental pressure sores in rats by biomechanical examinations. Lab Anim Res. 2012; 28(3):209–215. PMID:
23091522.

44. Babaei S, Bayat M, Nouruzian M, Bayat M. Pentoxifylline improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2013; 700(1-3):165–172. PMID:
23220163.

45. Babaei S, Bayat M. Pentoxifylline Accelerates Wound Healing Process by Modulating Gene Expression of MMP-1, MMP-3, and TIMP-1 in Normoglycemic Rats. J Invest Surg. 2015; 28(4):196–201. PMID:
26087281.

46. Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med. 1998; 22(2):97–102. PMID:
9484702.

47. Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003; 14(Suppl 3):S13–S18. PMID:
12730800.

48. Ammann P. [Determining factors of bone mechanical resistance]. Therapie. 2003; 58(5):403–407. PMID:
14682187.
49. Wakabayashi S, Tsutsumimoto T, Kawasaki S, Kinoshita T, Horiuchi H, Takaoka K. Involvement of phosphodiesterase isozymes in osteoblastic differentiation. J Bone Miner Res. 2002; 17(2):249–256. PMID:
11811555.

50. Rawadi G, Ferrer C, Spinella-Jaegle S, Roman-Roman S, Bouali Y, Baron R. 1-(5-oxohexyl)-3,7-Dimethylxanthine, a phosphodiesterase inhibitor, activates MAPK cascades and promotes osteoblast differentiation by a mechanism independent of PKA activation (pentoxifylline promotes osteoblast differentiation). Endocrinology. 2001; 142(11):4673–4682. PMID:
11606432.
51. McLeod NM, Pratt CA, Mellor TK, Brennan PA. Pentoxifylline and tocopherol in the management of patients with osteoradionecrosis, the Portsmouth experience. Br J Oral Maxillofac Surg. 2012; 50(1):41–44. PMID:
21247671.

52. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009; 122(2 Suppl):S14–S21.

53. Kolios L, Hoerster AK, Sehmisch S, Malcherek MC, Rack T, Tezval M, Seidlova-Wuttke D, Wuttke W, Stuermer KM, Stuermer EK. Do estrogen and alendronate improve metaphyseal fracture healing when applied as osteoporosis prophylaxis? Calcif Tissue Int. 2010; 86(1):23–32. PMID:
19949941.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download