This article has been corrected. See "Erratum: Comparison of humoral and cell-mediated immunity in three different C57BL/6N mouse substrains" in Volume 33 on page 316.
Abstract
Adaptive immunity is a type of immune response mediated by T and B cells, and is important response for immune response amplification and memory. In this study, the adaptive immunologic properties of C57BL/6NKorl substrain were compared with those of two other C57BL/6N substrains. There were no significant differences between the C57BL/6NKorl and the two other C57BL/6N substrains in the histological structures of the thymus and spleen, which are immunologic organs containing T cell and B cells. In addition, flow cytometric analysis did not reveal any significant differences in the distribution of T and B cell populations of the three substrains. To evaluate cell-mediated immunity of T cells in the three different substrains, we treated isolated T cells from spleen with Con A. The T cells of C57BL/6NKorl showed Con A-dependent proliferation of T cells at lower cell number than those in T cells from the other two C57BL/6N substrains. B cell-mediated humoral immune responses were not significant different among the three substrains. Thus, the results of this study provide evidence that C57BL/6NKorl mice are similar to those two other C57BL/6N substrains in humoral immunity, but C57BL/6NKorl has stronger response in cell mediated immunity.
Inbred mice provide researchers with experimental confidence in their animal-based experiments by providing the researcher with genetically homogeneous animals. Numerous inbred mouse types have been introduced since scientists first produced an inbred strain in the early 20th century [1]. Such mice exhibit various phenotypes are classified according to their respective mouse strain's characteristics, such as hair color, blood parameters, biological behavior, immune response, response to stress, disease susceptibility (e.g., atherosclerosis, diabetes mellitus, or cancer), gene knockout, and response to toxicity [23456789101112].
C57BL/6 mice were isolated in 1937 from the C57BL strain developed by C.C. Little and are maintained under the substrain name C57BL/6J in Jackson Laboratories. The C57BL/6N substrain was isolated from C57BL/6 mice at Jackson Laboratories in 1951 and the substrain is maintained at the NIH. Maintenance was later extended to various locations, such as the Charles River Laboratory [131415].
Differences in the immunological responses of inbred mice are important because immune responses to pathogens, cell damage, diseases, and transplantation are being widely studies. The immunological characteristics of C57BL/6 mice are relatively well described because, during early establishment of inbred mouse lines, the C57BL/6 substrain was developed for use in research into antitumor effects and immunological studies [16]. In general, the immune response can be divided into innate immunity and adaptive immunity, and adaptive immunity can be further divided into humoral or cell-mediated immunity. Innate immunity is broadly related to physiological barriers such as the pH of the stomach and anatomical barriers including the epithelial barrier. As well, the innate immune response is relatively immediate, phagocytes (particularly neutrophils and macrophages), natural killer cells, complement activation, and nonspecific defense. Adaptive immunity is more specific than innate immunity and is mediated by B and T lymphocytes. However, the boundaries between innate immunity and adaptive immunity are not always distinguishable or mutually exclusive. The immune response of C57BL/6 mice is characterized by the presence of adaptive immunity. In that strain, TH1 type T cells, which secrete cytokines that stimulate the cell-mediated immune response, are relatively predominant when compared with those in Balb/c mice. This predominance of Th1 cells suggests that the immune response of C57BL/6 mice is strongly involved cell-mediated immunity, and that the substrain's humoral immunity level is relatively low [17181920212223].
The C57BL/6NKorl substrain has been designated as an established inbred mouse substrain as in the mouse and rat strain nomenclature guidelines of the National Institute of Food and Drug Administration (NIFDS). Moreover, it has been verified that C57BL/6NKorl is a genetically distinct substrain from other C57BL/6N substrains. However, the immunological properties of C57BL/6NKorl substrain have not been compared with those of other C57BL/6N mice. In this study, by examing humoral and cell-mediated immune response, we investigated whether the immune response of C57BL/6NKorl displayed an adaptive immunity level that was equal, or perhaps superior, to those of two other C57BL/6N substrains.
The animal protocols used in this study were reviewed and approved by PNU-Institutional Animal Care and Use Committee (PNU-IACUC; approval number PNU-2016-1207). All C57BL/6N mice were maintained and treated at the Animal Resource Center of Animal Pusan National University, which was certified by the Food and Drug Administration (FDA) as accredited unit number 000231, and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International according to the National Institutes of Health guidelines (Accredited Unit Number; 001525). Six-week old male C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mice were obtained, and after being maintained in the in the animal room for one week, mice were sacrificed by using CO2 gas.
Spleen and thymus tissues were dissected from the euthanized mice and fixed with 4% neutral buffered formaldehyde (pH 6.8) for overnight, after which each tissue sample was dehydrated. The dehydrated sample was embedded in paraffin, and the paraffin block was then cut into 4 µm sections using a Leica microtome (Leica Microsystems, Bannockburn, IL, USA). The sections were deparaffinized with xylene, rehydrated with ethanol to a reduced concentration of 100 to 70%, and finally washed with distilled water. Slides bearing mounted spleen and thymus sections were stained with hematoxylin (Sigma-Aldrich St. Louis, MO, USA) and eosin (Sigma-Aldrich) and washed with dH2O. Pathological changes in tissue samples were examined by using the Leica Application Suite (Leica Microsystems).
For distribution and allo-antigenic analysis of immune cells, we performed flow cytometric analysis. Spleen was obtained by a general mouse dissecting method, after which, a portion of the spleen from each mouse was minced by using an iron mesh and a glass rod. Splenocytes were separated by using a centrifugation method. An RBC lysing buffer (Sigma-Aldrich) was used to remove erythrocytes and the pellet was suspended in complete RPMI media. Spleen cells were aliquoted in a medium volume of 300 µL (5×105/tube) and then incubated at room temperature for 20 min with ether T or B cells. Flow cytometric analysis was performed by using a Muse® cell analyzer (Millipore, Billerica, MA, USA).
Spleen cells were cultured in RPMI medium 1640 Glutamax I (Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; HyClone, Thermo Fisher Scientific, UK) and 1% (v/v) Penicillin/Streptomycin (Gibco) at 37℃ in a humidified 5% CO2 atmosphere. Spleen cells were seeded at 1×106 cells/well and 2×106 cells/well in a 96-well plate. To determine the immunotoxicity of spleen cells, lipopolysaccharide (LPS) or concanavalin A (Con A) were treated at predetermined concentrations (0.1, 0.5, 1, and 5 µg/well) and the cells cultured for 72 hours, after which WST-1 solution (Sigma-Aldrich) 20 µL was added, and the cells cultured for a further 4 hours. Changes in color in each well were measured at 450 nm by using a Soft Max Pro5 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Untreated cells served as a negative control. The experiments were performed in triplicate.
Significant differences between each groups were identified by performing one-way analysis of variance (ANOVA) as included in SPSS for Windows, release 10.10, Standard Version (SPSS., Chicago, IL, USA). All values are reported as the mean±standard deviation (SD) and a P value <0.05 was considered as significant.
In general, lymphoid organs may be divided into primary organs where immune cells develop and secondary organs where immune cells meet with an antigen. Bone marrow, a representative primary immune structure involved in the development of immune cells, produces a variety of immune cells ranging from red blood cells and platelets to macrophages, as well as T and B lymphocytes responsible for adaptive immunity from a hematological point of view. We analyzed immune system difference in the thymus, a primary immune organ that develops T cells, and the spleen, which develops T and B lymphocytes and has an important role in adaptive immunity. Figure 1 presents a representative histological view of the thymus. The tissue of the thymus is largely divided into the cortex and the medulla, and immature T cells called the thymocytes migrate from the cortex to the medulla. There were no significant differences in the size and histology of the thymous of among the C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mice. The spleen has a structure that presents as white and red pulps. There are lymphocytes in white pulp and red blood cells and macrophages in the red pulp. Similar to thymus, there were no histological differences among the spleen sample of C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mice (Figure 2).
To examine potential immunotoxicity differences among the three mouse substrains, the distributions of immune cells in spleen, which contains mature T and B cells was examined in the three substrains. Figure 3 shows the flow cytometric results for splenocytes after using antibodies against cell markers of T and B cells. There were no significant differences in the distribution of T and B cells obtained from the spleens of C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mice.
Cell-mediated immune responses are mediated by T cells, and in order to compare the activity of T cell-rendered cell-mediated immune response in three mouse substrains, splenic T cells were plated with varying numbers and treated with mitogen of T cells, which induce growth of T cells in spleen cells by dose dependent manner. Figure 4 shows that Con A induced a proliferation response of T cells in each substrain in a concentration-dependent manner. Figure 4C shows the T cell proliferation in C57BL/6NKorl cells, which was notably different from the results for C57BL/6NA and C57BL/6NB, respectively (Fig. 4A and 4B, respectively). The proliferation in C57BL6NKorl showed the Con A concentration-dependent proliferation pattern at a low cell concentration. These results suggest that the cell-mediated immune response of C57BL/6NKorl is stronger than that of the other two C57BL/6N substrains.
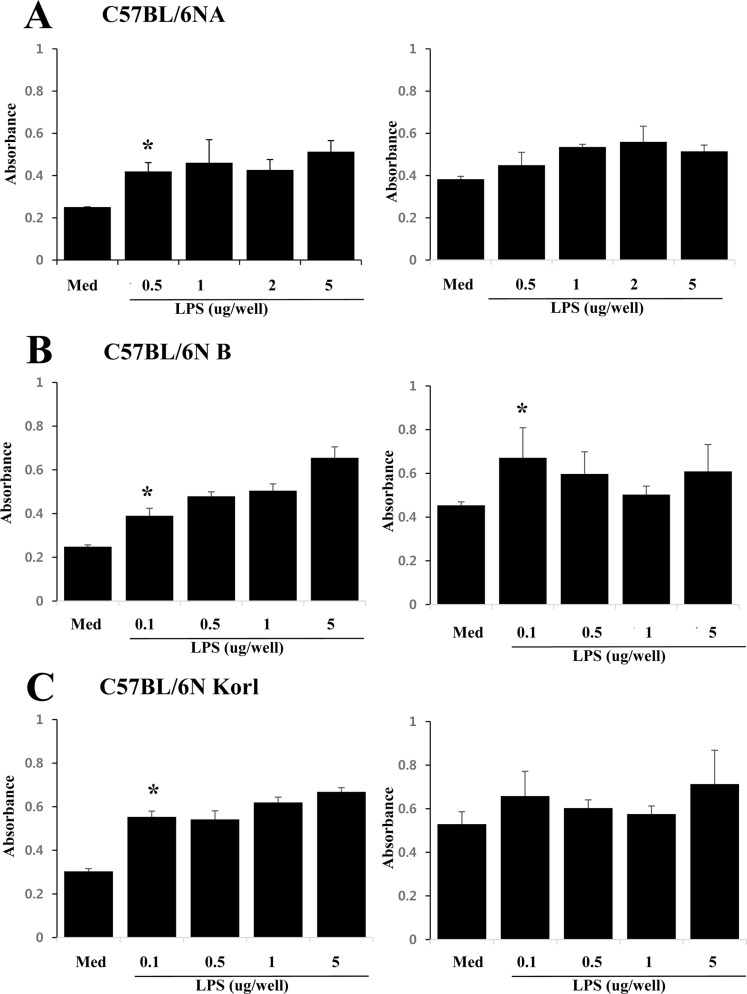
The antibody-mediated humoral immune response is driven by B cells producing antibodies. In order to measure the activity of the humoral immune system of each C57BL/6N substrains, the degree of proliferation of antibody-producing B cells was measured by using LPS, a mitogen that proliferates only B cells. Figure 5 shows the concentration-dependent B cell proliferation induced by LPS in each C57BL/6N substrains. All substrain showed similar B cell proliferation.
Inbred mouse strains are produced after 20 or more successive generations of sister-brother mating [24]. As is generally accepted, a substrain of the inbred strain must meet three conditions in order to be recognized as a genetically inbred strain that is different from the original inbred strain. The three conditions are: (1) mice obtained from 20th generation to 40th generation of the original strain, (2) mouse strain branch separated by more than 20 generations from the original strain, and (3) presence of genetic differences between branches [25]. C57BL/6NKorl is an inbred substrain established as a research result of Korean Food and Drug Administration which aimed to localize experimental animal sources. After meeting the above three conditions, the C57BL/6NKorl substrain code was registered and certified in 2015 by the Laboratory Animals Association (ILAR) under the National Institute of Health, which manages the codes of laboratory animal producers worldwide.
Differences in the immunological responses of inbred mouse substrains are important because substrains are widely used to assess responses to pathogens, cell damage, disease, and transplantation. In particular, C57BL/6 is a immunologically important substrain has been used in early development of inbred mice to research cancer and immune responses [26].
T cells and B cells, which are important for adaptive immunity, are developed and matured in bone marrow. B cells mature in the bone marrow, but T cells migrate to the thymus to mature and function. The mature T cell and B cell migrate to a secondary immune organ such as the spleen, where they may meet with an antigen, proliferate, and activate. Therefore, it is necessary to observe the histological structure of the spleen and the distribution of T cells and B cells within the spleen to derive an index for measuring an organism's level of adaptive immunity. Examination of the histological structures of C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mouse spleens showed no significant differences, and the distribution of T and B cells in those substrains were also similar.
Several studies have shown that C57BL/6 mice haves a higher activity of TH1 type T cells than SJL/J and Balb/c mice. In general, the function of helper T cells is to secrete cytokines and have a role in driving cell-mediated or humoral immune responses. Therefore, a higher TH1 type means that the C57BL/6N substrain has an enhanced cell-mediated immune response, whereas the substrain's humoral immune responses are likely to be lower than that in other mouse strains. Interestingly, C57BL/6NKorl exhibited a concentration-dependent proliferative function at lower cell concentrations than those of the other two C57BL/6N substrains. This suggests that C57BL/6NKorl has relatively high cell-mediated immune response activity. As expected, the humoral immune response was similar in the three C57BL/6N substrains.
In this study, we performed immunohistochemical analysis of the spleen and thymus, the distribution of immune cells, and the cell-mediated and humoral immunity in three C57BL/6N substrain. Thymus and spleen from the three C57BL/6N substrain showed similar immunohistochemical structures and similar distributions of T cells and B cells. However, our results showed that C57BL/6NKorl mice had greater cell-mediated immunity than those in the other two substrains. The humoral immune responses were similar in all three C57BL/6N substrains. Thus, our results suggest that the C57BL/6NKorl mice can be used in immunotoxicity studies as effectively as the C57BL/6NA and C57BL/6NB mice provided by commercial suppliers.
Acknowledgments
This project was supported by a grant of BIOREIN (Laboratory Animal Bio Resources Initiative) from Ministry of Food and Drug Safety in 2016.
References
1. Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat Genet. 2005; 37(11):1175–1180. PMID: 16254563.

2. Barros SF, Friedlanskaia I, Petricevich VL, Kipnis TL. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediators Inflamm. 1998; 7(5):339–346. PMID: 9883969.
3. Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl). 1997; 132(2):107–124. PMID: 9266608.

4. Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM Jr. Metabolic responses to leptin in obese db/db mice are strain dependent. Am J Physiol Regul Integr Comp Physiol. 2001; 281(1):R115–R132. PMID: 11404285.

5. Kile BT, Mason-Garrison CL, Justice MJ. Sex and strain-related differences in the peripheral blood cell values of inbred mouse strains. Mamm Genome. 2003; 14(1):81–85. PMID: 12532271.

6. Madiehe AM, Hebert S, Mitchell TD, Harris RB. Strain-dependent stimulation of growth in leptin-treated obese db/db mice. Endocrinology. 2002; 143(10):3875–3883. PMID: 12239099.
7. Nishina PM, Wang J, Toyofuku W, Kuypers FA, Ishida BY, Paigen B. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids. 1993; 28(7):599–605. PMID: 8355588.

8. Opsahl ML, McClenaghan M, Springbett A, Reid S, Lathe R, Colman A, Whitelaw CB. Multiple effects of genetic background on variegated transgene expression in mice. Genetics. 2002; 160(3):1107–1112. PMID: 11901126.

9. Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003; 52(8):1958–1966. PMID: 12882911.

10. Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol. 2012; 49(1):32–43. PMID: 22135019.
11. van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006; 5(2):139–149. PMID: 16507005.

12. Woodworth CD, Michael E, Smith L, Vijayachandra K, Glick A, Hennings H, Yuspa SH. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 2004; 25(9):1771–1778. PMID: 15105299.

13. Dux A, Mühlbock O, Bailey DW. Genetic analyses of differences in incidence of mammary tumors and reticulum cell neoplasms with the use of recombinant inbred lines of mice. J Natl Cancer Inst. 1978; 61(4):1125–1129. PMID: 212568.
14. Festing MF. Properties of inbred strains and outbred stocks, with special reference to toxicity testing. J Toxicol Environ Health. 1979; 5(1):53–68. PMID: 423306.

15. Zurita E, Chagoyen M, Cantero M, Alonso R, González-Neira A, López-Jiménez A, López-Moreno JA, Landel CP, Benítez J, Pazos F, Montoliu L. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011; 20(3):481–489. PMID: 20506040.

16. Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980; 65(5):929–935. PMID: 6933263.
17. Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol. 2011; 11(5):307–317. PMID: 21475308.

18. Chen J, Flurkey K, Harrison DE. A reduced peripheral blood CD4(+) lymphocyte proportion is a consistent ageing phenotype. Mech Ageing Dev. 2002; 123(2-3):145–153. PMID: 11718808.

19. Chen J, Harrison DE. Quantitative trait loci regulating relative lymphocyte proportions in mouse peripheral blood. Blood. 2002; 99(2):561–566. PMID: 11781239.

20. Glineur S, Antoine-Moussiaux N, Michaux C, Desmecht D. Immune depression of the SJL/J mouse, a radioresistant and immunologically atypical inbred strain. Immunobiology. 2011; 216(1-2):213–217. PMID: 20965099.
21. Mayer A, Lilly F, Duran-Reynals ML. Genetically dominant resistance in mice to 3-methylcholanthrene-induced lymphoma. Proc Natl Acad Sci U S A. 1980; 77(5):2960–2963. PMID: 6930679.

22. McVicar DW, Winkler-Pickett R, Taylor LS, Makrigiannis A, Bennett M, Anderson SK, Ortaldo JR. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J Immunol. 2002; 169(4):1721–1728. PMID: 12165492.

23. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000; 164(12):6166–6173. PMID: 10843666.

24. Green MC, Grueneberg H, Hertwig P, Heston WE, Lyon MF, Medvedev NN, Snell GD, Staats J. A revision of the standardized genetic nomenclature for mice. J Hered. 1963; 54:159–162. PMID: 14057864.
25. Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE. Mouse Genome Database Group. The mouse genome database (MGD): new features facilitating a model system. Nucleic Acids Res. 2007; 35(Database issue):D630–D637. PMID: 17135206.

26. Mouse genetics: concepts and applications. New York: Oxford University Press;1995. p. 3–31.
Figure 1
Histological difference of thymus structure among C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mice. Thymus tissues were stained with H&E. Upper line displayed at 100× magnification. Bottom line was viewed at 40× magnification. Arrow, cortex; arrow head, medulla.
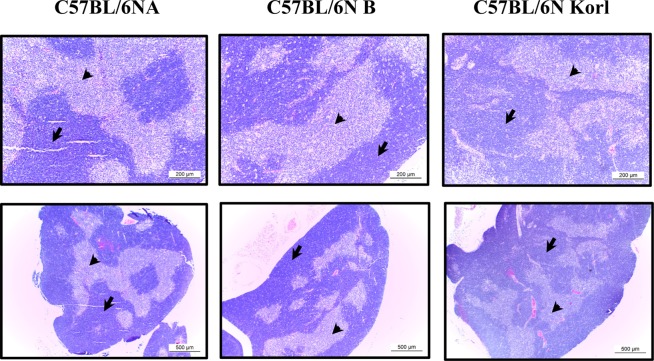
Figure 2
Histological difference of spleen structure among C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mice. Spleen tissues were stained with H&E. Upper line displayed at 100× magnification. Bottom line was viewed at 40× magnification. Arrow, Red pulp; arrow head, white pulp and lymphoid follicle.
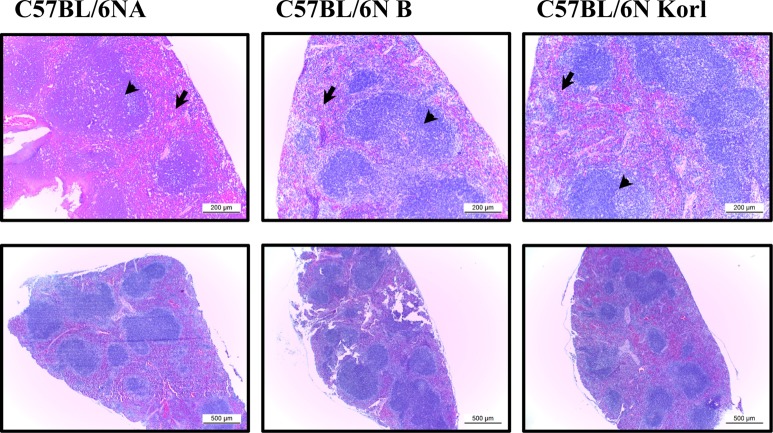
Figure 3
The relative level of lymphocyte population in C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mice. Histogram plots from flow cytometry represent the populations of T cells (left panel) and B cells (right panel) from spleen. Results are representative of 3 independent experiments.
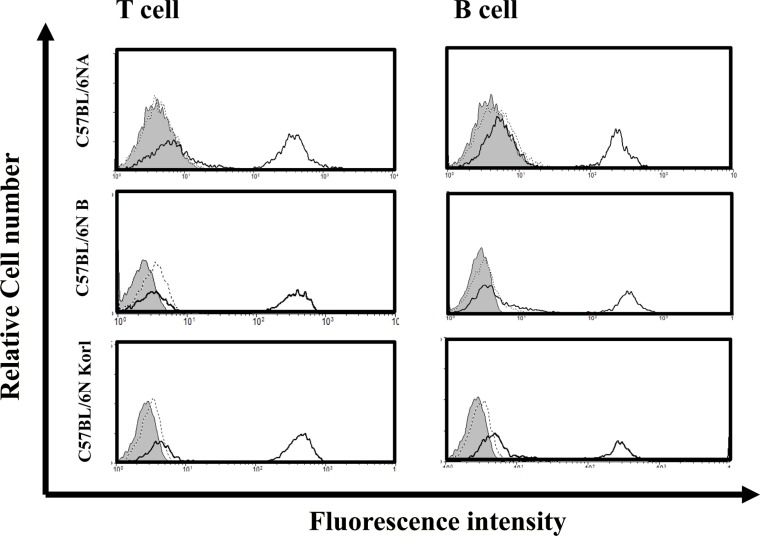
Figure 4
Determination of cell-mediated immunotoxicity. After treatment with different Con A doses, the growth of cells was measured by using WST-1 assays. The data are presented as means±SD of three experiments. (A) and (B) panels was display responses of splenic T cells from C57BL/6NA and C57BL/6NB substrain, respectively. (C) The data presented the response of splenic T cells from C57BL/6NKorl substrain. *P<0.05 vs. med group. Med, media-treated group; Con A, concanavalin A.
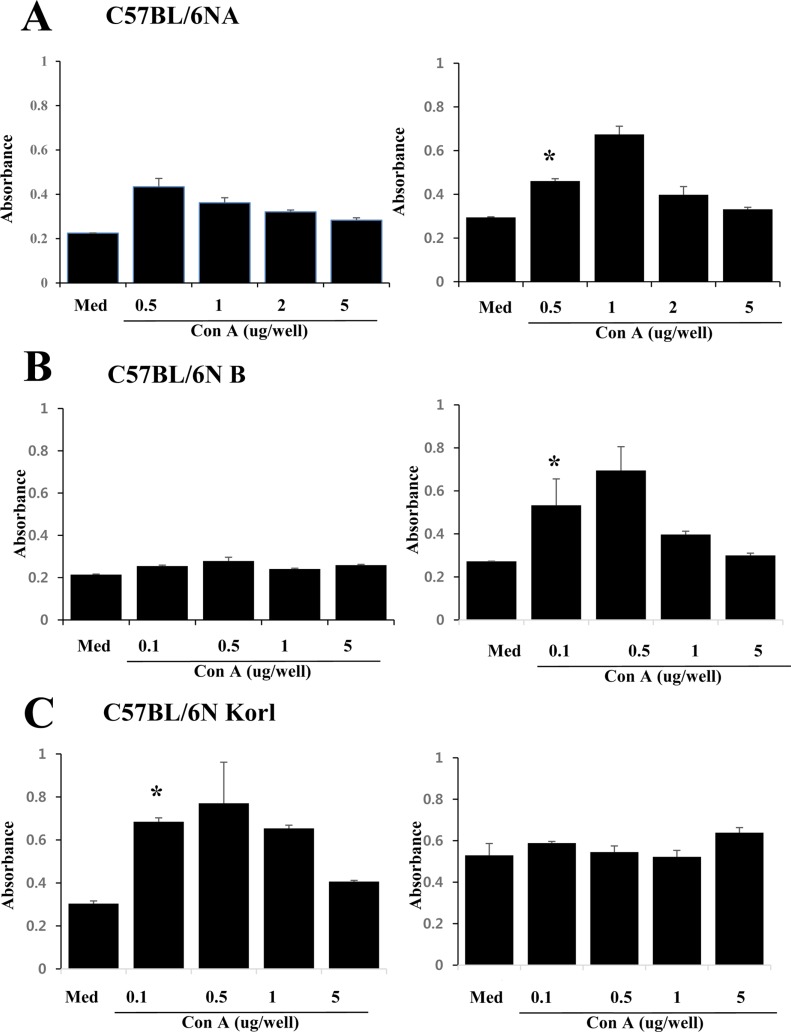
Figure 5
Determination of humoral immunotoxicity. After treatment with different LPS doses, the growth of cells was measured by using WST-1 assays. The data are presented as the means±SD of the experiments. (A) and (B) panels display response of splenic B cell from two different source C57BL/6N substrain. (C) The data presented response of splenic B cells from C57BL/6NKorl substrain. *P<0.05 vs. med group. Med, media-treated group; LPS, lipopolysaccharide.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download