This article has been corrected. See "Erratum: Comparative study of the immunological characteristics of three different C57BL/6N mouse substrains" in Volume 33 on page 315.
Abstract
Inbred mice, a systematically developed homogeneous animal, have been developed to maintain experimental reproducibility and to minimize experimental variables in animal-based studies. In particular, C57BL/6 mice are frequently used in experiments into immunology and antitumor activity experiments. This study was compared the immunological characteristics of C57BL/6NKorl, a Korean developed experimental animal resource, with those of two other C57BL/6N substrains. Mouse body, thymus, and spleen weights in C57BL/6NKorl were not significantly different from those of the other two C57BL/6N substrains. Among the three substrains, there was no difference in the distribution of T and B cells, which are lymphocytes involved in adaptive immunity, and no difference in NK cells related to innate immunity. Results for macrophages and granulocytes, which have roles in innate immunity, were similar in all three substrains. In order to investigate the expressions of major histocompatibility complex (MHC) molecules and allogenic antigens, splenocytes were separated from obtained spleen and analyzed by using flow cytometry. MHC class I and II molecules, which are important during self/non-self-discrimination, were similar in the three substrains. In addition, expression of alloantigen involved in allografts showed similar results in the three substrain. Thus, the results of this study provide strong evidence that C57BL/6NKorl is immunologically similar to two other C57BL/6N substrains.
Inbred mouse strains are established after twenty or more consecutive generations of sister-brother mating [1]. Initially, the establishment of inbred mouse was related to cancer and immunology studies and many of the early-established inbred strains, such as C57BL/6, C57BL/10, C3H, CBA, and BALB/c have important roles in all areas of biomedical research as they provide a standardized animal model that enables independent researchers scattered around the world to perform reproducible experiments on the same material [2]. In particular, C57BL/6 mice are one of the more well-known inbred substrains and were derived from the C57BL strain along with substrain C57BL/10. C57BL was established by C.C. Little in the 1920s and has been widely used as a universal strain and as a genetic background for spontaneous or induced mutant mice. The separation of substrains C57BL/6 and C57BL/10 occurred in the 1930s. Two well-described substrains are C57BL/6J and C57BL/6N, which were developed by the Jackson laboratory and the National Institutes of Health in the 1940s and 1950s, respectively, on the ancestral C57BL/6 line [34].
C57BL/6 mice have been used since the early days of inbred strain establishment studies in antitumor and immunological studies [5]. Therefore, many of the basic immunological properties of C57BL/6N mice have been described. In C57BL/6N mice, the activity of NK cells is relatively high, as is the activation of helper T cells, such as TH1, which secrete cytokines to promote cell-mediated immunity. Moreover, the C57BL/6N humoral immune response is lower than that of Balb/c mice [67891011].
To establish the C57BL/6NKorl substrain as a locally available, experimental animal source, research to obtain Food and Drug Administration registration was undertaken. The C57BL/6NKorl substrain was established after inbreeding for more than 20 generations. C57BL/6NKorl mice were initially identified as C57BL/6J. However, the name was changed to C57BL/6N after confirming that an Nnt gene deletion did not occur and that there was genetic differentiation from other cultivars. The presence of four genetic single nucleotide polymorphisms (SNP) in the C57BL/6NKorl mice were deemed unique within the C57BL/6N substrain and in 2015, the C57BL/6NKorl code was registered and certified by the Laboratory Animals Association (ILAR) within the National Institutes of Health, which manages the world's producer of laboratory animal producer. However, the immunological characteristics such as the distribution of immune cells, expressions of MHC class molecules, and expression of alloantigens in C57BL/6NKorl mice have not been fully described.
This study examines the distribution of immune cells, the expression of MHC class molecules, and the expression of alloantigens to transplantation in C57BL/6NKorl mice. The results demonstrate the usefulness of this Korean locally developed experimental animal resource in immunological studies.
The animal protocol used in this study, based on the ethical procedures for scientific care set by the PNU-Institutional Animal Care and Use Committee (PNU-IACUC) was reviewed and approved (Approval Number PNU-2016-1207). Six-week old male C57BL/6NKorl mice and same age and sex mice from two different origins of C57BL/6N substrains were maintained and handled in the PNU Laboratory Animal Resources Center, which is accredited by the Korean Food and Drug Administration (FDA; accredited unit number 000231) and the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International in accordance with National Institutes of Health guidelines (accredited unit 001525). During the experiments, the mice were maintained in a specific-pathogen-free (SPF) state under a strict light cycle (lights on at 08:00 h and off at 20:00 h) at 23±2℃ and 50%±10% relative humidity without any environmental enrichments. After being kept in the animal room for one week, the mice were sacrificed with CO2 gas. Body and organ weights were obtained by using an electronic balance (Mettler Toledo, Greifensse, Switzerland). Dissection was performed according to KFDA guidelines.
The body weights of mice from the three different C57BL/6N substrains and the weights of their immune organs (thymus and spleen) measured by using an electric balance. Thymus and spleen were removed following euthanasia. Weighing was performed three times to ensure accuracy.
Mouse immune cells were obtained from the spleen of mice and were obtained by following general mouse dissecting methods. Isolation of spleen cells was performed by mincing with iron mesh and glass rod followed by centrifugation. RBC lysing buffer (Sigma-Aldrich., St Louis, MO, USA) was used to remove erythrocytes, after which spleen cells were suspended in complete RPMI media. Antibodies were purchased from BioLegend or eBioscience. Cells were stained with CD3, CD19, F4/80, Gr-1 or NK1.1, and Flow cytometry (FACS) analysis was performed to determine the distribution of T cells, B cells, macrophages, granulocyte, and NK cells. MHC class I and II molecule and alloantigen expressions in spleen cells were determined using antibodies specific for H-2Db, H-2Kb, I-Ab, CD45, CD45.2, CD90, or CD90.2. Antibody stained cells were incubated for 30 min on ice bath. Cells were washed with PBS. Flow cytometry analysis was performed by using a Muse® cell analyzer (Millipore) and the results were analyzed by using WinMDI software.
Body weight of an experimental animal is important because it can indicate the animal's nutritional and health status. In addition, size and weight of secondary lymphoid organs, such as spleen, and primary lymphoid organs, such as bone marrow and thymus, as well as changes in external shape, are indicators of the status of the basic immune response in experimental animals. To examine the differences in the immune responses of the three C57BL/6N substrain mice, we compared the body, thymus, and spleen weights of the three C57BL/6N substrains (Figure 1). Neither the body weights nor the weights of spleen and thymus of the three C57BL/6N substrain were significantly different.
In the lymphoid lineage, T and B cells are involved in adaptive immunity and a third lymphocyte, NK cells, is involved in innate immunity. Figure 2 shows the distribution of these in the spleens of each the three mouse substrains obtained by performing flow cytometric analysis. The distributions of the T, B, and NK cells did not show any significant differences among the three substrains.
The myeloid lineage cells involved in innate immunity are mainly macrophages and granulocytes (neutrophils, eosinophils, basophils), which are immunologically important as the first line of defense against non-specific, immediate reactions to exogenous materials. The comparison of the distributions of macrophages and granulocytes in the three C57BL/6N mouse substrains failed to detect any significant differences (Figure 3).
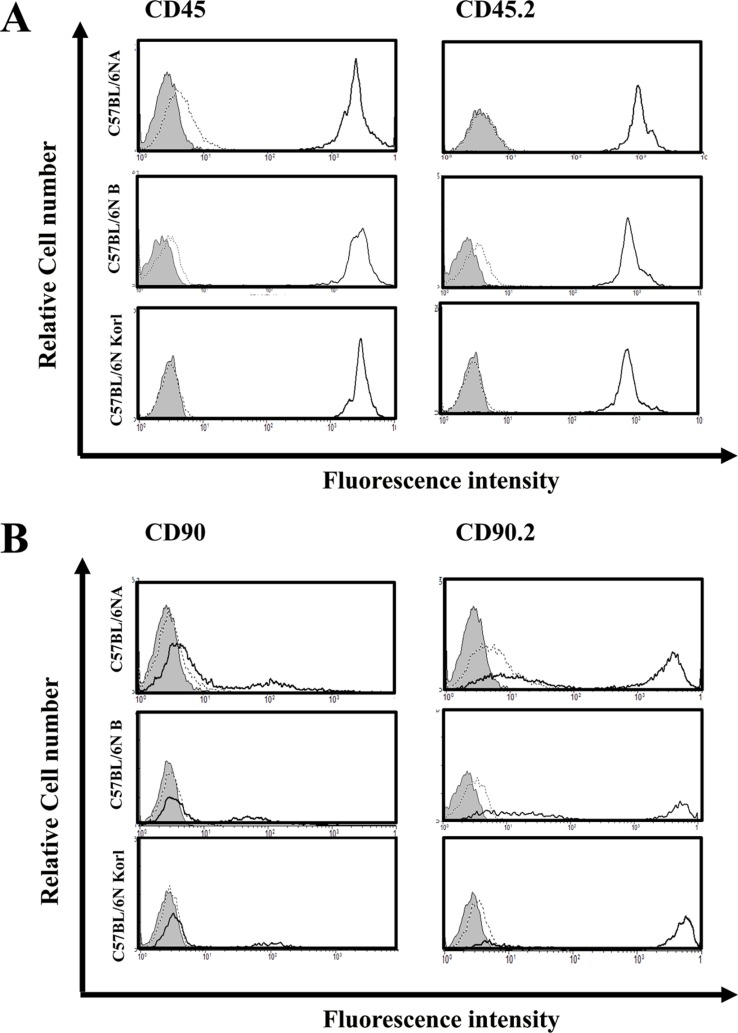
MHC molecules are immunologically important molecules that provide basic information about self/non-self-recognition of antigens presenting. MHC class I and class II molecules were compared among the three C57BL/6N substran. Mice from the three C57BL/6N substrain showed similar expression levels of both MHC class I and class II molecules (Figure 4). Alloantigen has an important role in allogeneic transplantation, is important in transplantation, and is useful in animal experiments into bone marrow transplantation and markers for distinguishing between immune cells of both recipient and donor. Figure 5 shows that all three C57BL/6N substrain had similar alloantigen expression patterns.
There are many reasons to use mice as an experimental animal, such as their short lifespan, excellent reproductive ability, and ease of breeding, thus they are widely used in various research fields including oncology, pharmacology, genetics, immunology and endocrinology. In particular, inbred mice achieved by generation of twenty or more consecutive generations of sister-brother crosses, produce offspring nearly identical genotypes [1]. Reproducibility is very important for biological experiments, and consistency of experimental animal characteristics is also important. The earliest established inbred mouse strains were developed for cancer and immunology studies, and the most well-described inbred strains, such as C57BL/6, C57BL/10, C3H, CBA, and BALB/c strains, have had important roles in all areas of biomedical research as they provide standardized animal models that allow independent researchers scattered around the world to perform reproducible experiments with the same animal strain [2].
Knowledge of the mouse genome has increased since genome sequencing was developed. In the early 20th century scientists produced the first inbred mouse strain, and a number of additional mouse types have been introduced since then [12]. Such mice are classified according to their respective mouse stains, such as hair color, blood parameters, biological behavior, immune response, response to stress, disease susceptibility (e.g., atherosclerosis, diabetes mellitus, and cancer), gene knockout, and a variety of other traits [1314151617181920212223].
The immune organs are divided into two major categories: primary and secondary immune organs. The primary immune organs are those where immune cells are formed and matured, such as bone marrow and thymus. The secondary immune organs are sites to which matured immune cells migrate, such as spleen and lymph nodes. Among the primary immune organs, the thymus is the site where T cells mature. In the case of the spleen and lymph nodes, which are secondary immune organs, their size and weight increase when an inflammatory reaction occurs. Therefore, the appearance, size, and weight of an immune organ can be regarded as a good indicator of the degree of an immune response. In this study, the weights of thymus and spleen of C57BL/6NKorl and two other C57BL/6N substrains did not show any significant differences when all were raised in same, well-controlled environment.
The immune response that is generated after the initiation of a pathogenic infection is called the innate immune response. Innate immunity has nonspecific, immediate barrier characteristics, such as anatomical barriers (including skin) and physiological barriers (including stomach pH). In addition, the immune cells of the innate immune response consist of macrophages or dendritic cells, which mainly recognize the pathogen, and natural killer cells and granulocytes (neutrophils, eosinophils, and basophils). Adaptive immunity is the reaction that serves to remove pathogens that have not been removed by this innate immune response and/or to inhibit the spread of a pathogenic infections. Adaptive immunity is more specific and associated with immune memory responses. The cells involved in adaptive immunity are B and T lymphocytes. These cells are known to induce a humoral immune response in order to produce antibodies and a cellular immune response to kill infected cells. The cellular immune response can be divided further to CD4 T lymphocytes (helper lymphocytes), which help to improve functional ability of immune cells by producing cytokines and CD8 T lymphocytes (cytotoxic lymphocytes), which induce infectious cell killing. Appropriate immunological distribution of each immune cell type responsible for innate immunity and adaptive immunity is important as their distribution can be used as an index for evaluating the immune response of experimental animals. In the present study, the immune cell distributions of the C57BL/6NKorl and the two other C57BL/6N substrains were similar.
Because inbred mice are genetically homogeneous, they are particularly useful in highly focused transplantation immunology studies. In transplant immunology, the expression of MHC molecules, it is necessary to verify the role of separating the self and the non-self, and information should be obtained by measuring the expression of alloantigen for allograft studies. Expressions of MHC molecules and alloantigen in the C57BL/6NKorl and the two other C57BL/6N substrain did not differ significantly.
In this study, there were no significant differences among the body weights and weights of immune organs, the distributions of immune cells, and the expressions of MHC molecules and alloantigen in three different substrain of C57BL/6N mice. Thus, our results suggest that C57BL/6NKorl mice can be used as extensively as other C57BL/6N mice that are provided by commercial suppliers for immunological studies.
Acknowledgments
This project was supported by a grant of BIOREIN (Laboratory Animal Bio Resources Initiative) from Ministry of Food and Drug Safety in 2016.
References
1. Green MC, Grueneberg H, Hertwig P, Heston WE, Lyon MF, Medvedev NN, Snell GD, Staats J. A revision of the standardized genetic nomenclature for mice. J Hered. 1963; 54:159–162. PMID: 14057864.
2. Silver LM. Mouse genetics : concepts and applications. New York: Oxford University Press;1995. p. 3–31.
3. Dux A, Mühlbock O, Bailey DW. Genetic analyses of differences in incidence of mammary tumors and reticulum cell neoplasms with the use of recombinant inbred lines of mice. J Natl Cancer Inst. 1978; 61(4):1125–1129. PMID: 212568.
4. Festing MF. Properties of inbred strains and outbred stocks, with special reference to toxicity testing. J Toxicol Environ Health. 1979; 5(1):53–68. PMID: 423306.

5. Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980; 65(5):929–935. PMID: 6933263.
6. Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol. 2011; 11(5):307–317. PMID: 21475308.

7. Chen J, Flurkey K, Harrison DE. A reduced peripheral blood CD4(+) lymphocyte proportion is a consistent ageing phenotype. Mech Ageing Dev. 2002; 123(2-3):145–153. PMID: 11718808.

8. Chen J, Harrison DE. Quantitative trait loci regulating relative lymphocyte proportions in mouse peripheral blood. Blood. 2002; 99(2):561–566. PMID: 11781239.

9. Glineur S, Antoine-Moussiaux N, Michaux C, Desmecht D. Immune depression of the SJL/J mouse, a radioresistant and immunologically atypical inbred strain. Immunobiology. 2011; 216(1-2):213–217. PMID: 20965099.

10. McVicar DW, Winkler-Pickett R, Taylor LS, Makrigiannis A, Bennett M, Anderson SK, Ortaldo JR. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J Immunol. 2002; 169(4):1721–1728. PMID: 12165492.

11. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000; 164(12):6166–6173. PMID: 10843666.

12. Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat Genet. 2005; 37(11):1175–1180. PMID: 16254563.

13. Barros SF, Friedlanskaia I, Petricevich VL, Kipnis TL. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediators Inflamm. 1998; 7(5):339–346. PMID: 9883969.
14. Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl). 1997; 132(2):107–124. PMID: 9266608.

15. Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM Jr. Metabolic responses to leptin in obese db/db mice are strain dependent. Am J Physiol Regul Integr Comp Physiol. 2001; 281(1):R115–R132. PMID: 11404285.

16. Kile BT, Mason-Garrison CL, Justice MJ. Sex and strain-related differences in the peripheral blood cell values of inbred mouse strains. Mamm Genome. 2003; 14(1):81–85. PMID: 12532271.

17. Madiehe AM, Hebert S, Mitchell TD, Harris RB. Strain-dependent stimulation of growth in leptin-treated obese db/db mice. Endocrinology. 2002; 143(10):3875–3883. PMID: 12239099.
18. Nishina PM, Wang J, Toyofuku W, Kuypers FA, Ishida BY, Paigen B. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids. 1993; 28(7):599–605. PMID: 8355588.

19. Opsahl ML, McClenaghan M, Springbett A, Reid S, Lathe R, Colman A, Whitelaw CB. Multiple effects of genetic background on variegated transgene expression in mice. Genetics. 2002; 160(3):1107–1112. PMID: 11901126.

20. Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003; 52(8):1958–1966. PMID: 12882911.

21. Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol. 2012; 49(1):32–43. PMID: 22135019.
22. van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006; 5(2):139–149. PMID: 16507005.

23. Woodworth CD, Michael E, Smith L, Vijayachandra K, Glick A, Hennings H, Yuspa SH. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 2004; 25(9):1771–1778. PMID: 15105299.

Figure 1
Body, spleen, and thymus weights in C57BL/6NKorl mouse compared to those in two other C57BL/6N mouse substrains. Weights were determined at 7 weeks of age. each mouse was measured three times (n=3).
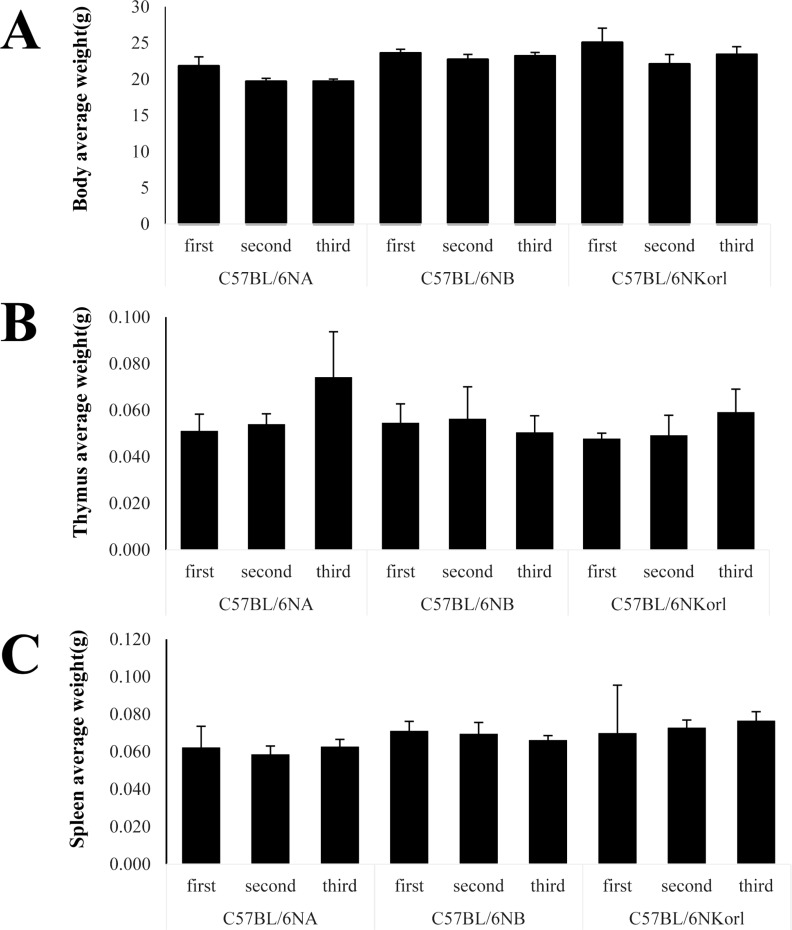
Figure 2
Relative levels of lymphocyte populations in C57BL/6NKorl mouse and two C57BL/6N substrain. (A) Histogram plots from flow cytometry present the population of T cells (left panel) and B cells (right panel) from spleen. (B) Histogram plot displays the total NK cell population in spleen cells. Results are representative of 3 independent experiments.
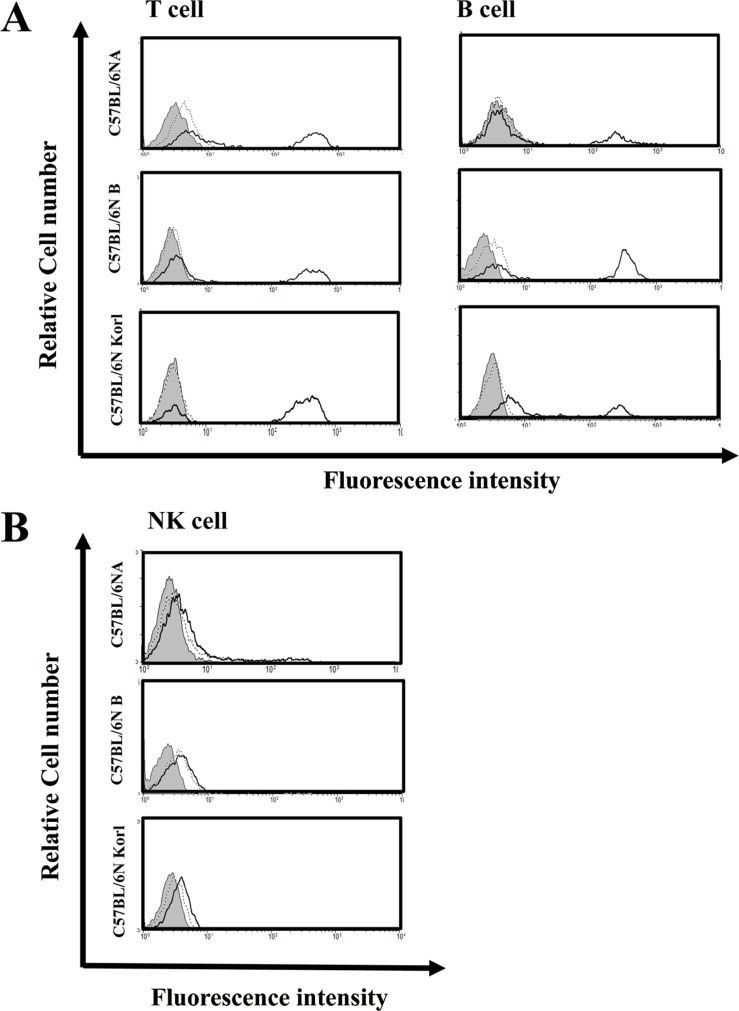
Figure 3
Relative levels of myeloid lineage cell populations in C57BL/6NKorl mouse and two other C57BL/6N substrains. Histogram plots of flow cytometry presented the population of macrophages (left panel) and granulocytes (right panel) from spleen. Results are representative of 3 independent experiments.
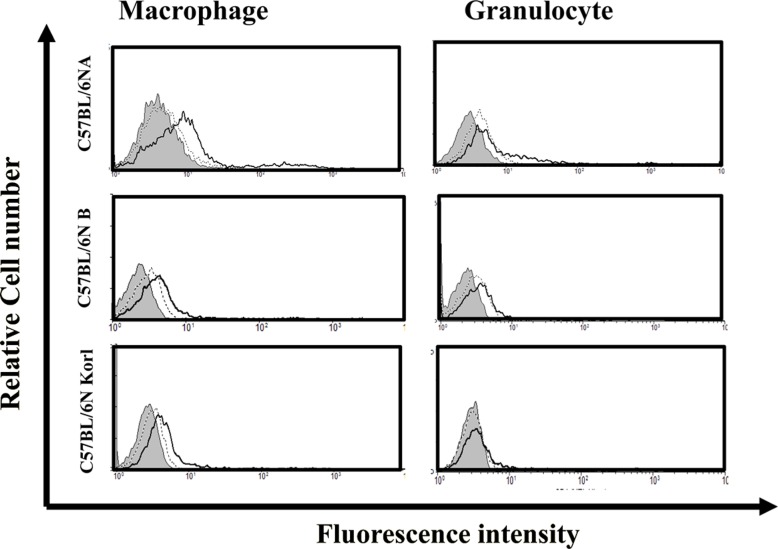
Figure 4
Determination of mouse major histocompatibility complex (MHC) molecules in C57BL/6NKorl mouse and two other C57BL/6N substrains. (A) Histogram plots of flow cytometry presented the expression of MHC class I molecule on spleen cells. Left histogram plot illustrates the expression level of H-2Db on spleen cells. Right histogram plot displays the expression level of H-2Kb on spleen cells. (B) Histogram plot displays the expression level of MHC class II, I-Ab on spleen cells. Results are representative of 3 independent experiments.
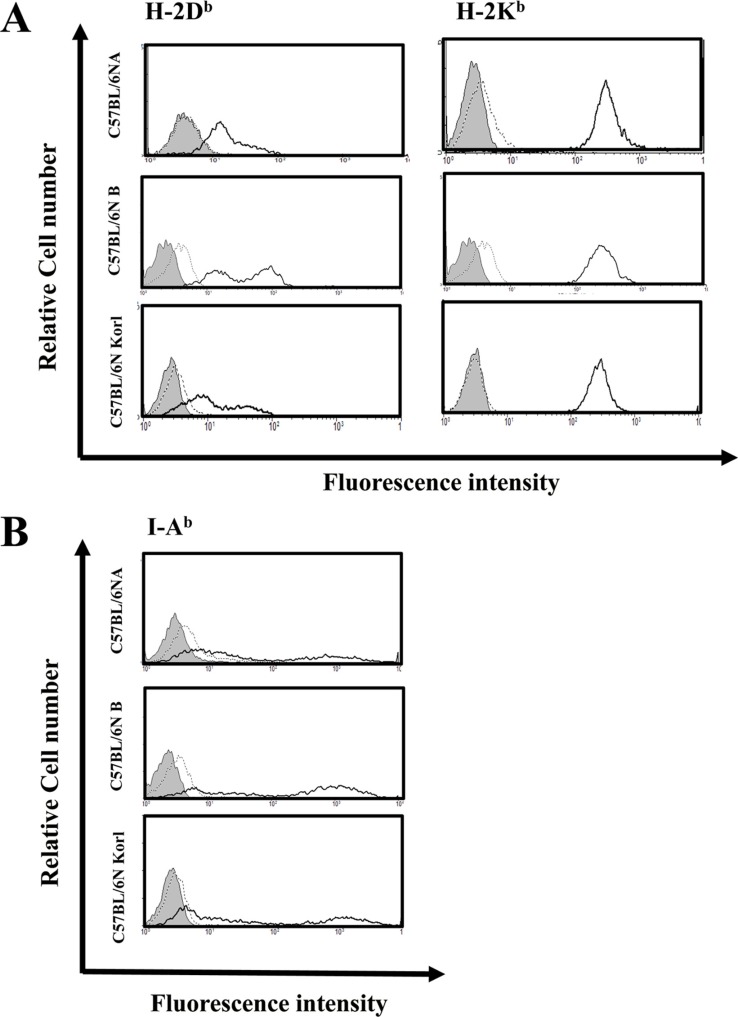
Figure 5
The expression levels of mouse alloantigen in C57BL/6NKorl mouse and two other C57BL/6N substrains. (A) Histogram plots presented the expression of CD45 (left panel) and CD45.2 (right panel) on spleen cells. (B) Left histogram plot illustrates the expression level of CD90 on spleen cells. Right histogram plot displays the expression level of CD90.2 on spleen cells. Results are representative of 3 independent experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download