Abstract
Inbred mice are an essential animal strain for research as they can improve the reproducibility and reliability of study results. The establishment of new inbred lines is continuing, and new inbred lines are being used in many research fields. C57BL/6 is a mouse laboratory animal that has been developed and used as an inbred strain since early stage of mouse strain development, and, in the 1950s, C57BL/6 was separated into substrains by the Jackson Laboratory (C57BL/6J) and the National Institutes of Health (C57BL/6N). C57BL/6 mice have been used in immunology and antitumor activity studies since the early strain development stage. After the mouse genome was fully described, C57BL/6 mice use in many areas of research has expanded. In particular, immunological characteristics such as those related to cell-mediated immunity and NK cell activity are relatively higher in C57BL/6 mice than in other mice. The C57BL/6NKorl is a stock of C57BL/6N established as part of a localization of experimental animal strategy of the Korean Food and Drug Administration. Based on analysis of single nucleotide polymorphisms (SNPs), C57BL/6NKorl is considered a genetically distinct inbred stock from other C57BL/6N. Various research efforts have been made to describe the characteristics and increase knowledge of the characteristics of C57BL/6Nkorl. The results obtained through these efforts are expected to increase the utilization of C57BL/6Nkorl as a domestic laboratory animal resource and to enhance the reliability of mouse based studies.
Experimental animal strains are useful animal resources. Such animal strains are generally involved in biomedical and behavioral experiments, and their use in experiments has contributed substantially to describing and elucidating biological mechanisms and metabolic pathways [1]. Specifically, inbred animal strains have been favored by researchers for improving the reliability and reproducibility of animal-based experiments. The origin of the inbred strain begins with the inbred mating experiment of guinea pig (Cavia porcellus) in 1906 by G. M. Rommel [2], and, since that time, various inbred strains of laboratory animals have been used. Among them, laboratory mice are preferred due to their short life span and ease of breeding, and a number of currently inbred mouse strains (e.g., C3H, C57BL/6 and CBA) have been developed. In addition, mouse genome sequencing has been completed, and the availability of such information has attracted much attention. Since the early 20th century when the first inbred mouse strain was established, a number of mouse types have been introduced [3]. Such mice are classified according to their respective mouse strains characteristics, such as hair color, blood parameters, biological behavior, immune response, response to stress, disease susceptibility (e.g., atherosclerosis, diabetes mellitus and its complications, and cancer), gene knockout, and a variety of trait-specific responses (Table 1) [4567891011121314].
Inbred mouse strains are produced after completion of 20 or more consecutive generations of sister-brother mating system [15]. Early inbred strains were developed for cancer and immunology studies, including some of the more famous early inbred hybrids; for example, C57BL/6, C57BL/10, C3H, CBA and BALB/c strains. Such inbreeding and the resultant variety of inbred strains have important roles in all areas of biomedical research by providing standardized animal models that enable independent researchers scattered around the world to obtain reproducible experimental results using the same material [16].
The C57BL mouse was established by C.C. Little in the 1920s and has been widely used as a universal stain and as a generic genetic background source of spontaneous or induced mutant mice. The C57BL/6 mouse strain is one of the more widely known inbred strains derived from the C57BL. Another widely known strain developed from C57BL is C57BL/10. The separation of C57BL/6 and C57BL/10 occurred in the 1930s. From the C57BL/6 strain, two of the more prominent substrains are C57BL/6J and C57BL/6N, which were developed by the Jackson Institute (JAX, Bar Harbor, Maine, USA) and the National Institutes of Health (NIH, Bethesda, MD, USA), respectively, in the 1940s and 1950s [117]. Substrains of the original inbred strain must meet three conditions in order to be recognized as a genetically different inbred substrain from the original inbred strain. First, the substrain is derived from 20th to 40th generation inbred mice. Second, generation branches are to be separated by more than 20 generations from the common ancestor. Third, there is to be a genetic difference between generation branches [18].
In the 1950s, C57BL/6J substrain mice were transferred from JAX to NIH and maintained by the NIH for several decades to produce a separate substrain known as C57BL/6N. Some C57BL/6N mice were then sent to commercial distributors Charles River Laboratories (Worcester, MA, USA) to establish C57BL/6NCrl, Harlan Laboratories (Latham Drive, Madison, WI, USA) to establish C57BL/6NHsd and Taconic Biosciences (Hudson, NY, USA) to establish C57BL/6NTac as well, some were sent to JAX to establish C57BL/6NJ (Figure 1). As required by the definition of a substrain, significant genetic variation may occur between these substrain. However, single nucleotide polymorphism (SNP) genotyping panels failed to detect a significant genetic difference among the Harlan, Taconic, or Charles River 6N substrains [1920]. Of course, current genetic testing has limitation, so a difference may not be detected. Therefore, it is necessary to use a substrain that is suitable for the research purpose and to use the same supply company in order to ensure the homogeneity of experimental animals.
Differences in the immunological responses of inbred mouse are of interest because they can be used to assess responses to pathogens and cell damage related to diseases and transplantation. C57BL/6 mice, in particular, are notable for their immunogenicity due to the initial strain development goal being their use in the study of cancer and immune responses. In general, the immune response is divided into an innate immunity and an adaptive immunity, and adaptive immunity is divided further into humoral and cell-mediated immunity. Innate immunity is broadly related to physiological barriers, such as the pH of the stomach, and anatomical barriers, such as the epithelial barrier and relatively immediate-acting phagocytes, natural killer cells, and the complement activation nonspecific defense system. Adaptive immunity is more specific than innate immunity and is mediated by B and T lymphocytes. However, the boundaries between innate immunity and adaptive immunity are not always distinguishable or mutually exclusive.
Cells associated with innate immunity include granulocytes neutrophils, eosinophils neutrophils, macrophages, and NK cells [21]. In general, these unique immune-related cells trigger nonspecific and acute immune responses, such as the onset of inflammatory reactions, and have been reported have major cellular roles. Moreover, their distribution within the immune system is important. Unlike myeloid lineage cells, NK cells have a lymphoid lineage and have an important role to function in antiviral and anti-tumoral responses and are particularly important as the primary donor of the interferon gamma cytokine [22].
Among the receptors that have important roles in the function of NK cells are killer cell lectin-like receptors (KLRAs) in humans. The Ly49 receptor in mice exhibits differences in specific receptor expression among inbred mice. For example, Ly49O and Ly49P are expressed in 129/J mice, whereas Ly49L is expressed in CBA/J and C3H/He mice. None of these are expressed in C57BL/6 mice. however, C57BL/6 mice do express receptors like Ly49H, and the differences in expression of these Ly49 receptors may contribute to differences in the activation of NK cells against tumors and viruses [232425].
As described above, C57BL/6 mice have been used since the early days of inbred strain establishment in studies related to response to antitumor and immunological response [26]. It is well known that the activity of NK cells, which have an important role in antitumor activity, is relatively high in C57BL/6 mice. C57BL/6 mice are also resistant to mouse cytomegalovirus through their expression of the LY49 receptor. These reports suggest that activation signals produced by NK cells may have an important role in the immune response differences between inbred strains. Results of activated receptor studies in rats are important when considering in the production of mutant mice for use in antitumor activity and immune studies [2728].
Adaptive immune responses are linked to native immunity, and lymphoid lineage B and T lymphocytes and their subtype cells are involved in adaptive immunity. For example, C57BL/6, unlike SJL/J, reverses the B:T cell ratio, suggesting that the ratio of B lymphocytes to T lymphocytes circulating in C57BL/6 mice is inversely proportional to other inbred strains such as SJL/J [29303132].
Cell-mediated T-lymphocytes consist of mediate T-helper cells, cytotoxic T cells, memory T cells, and other subtypes [3334]. Helper T cells are divided into TH1 and TH2 cells according to their mode of cytokine expression, which ultimately provides the immune response to the cell-mediated or to the humoral immune response by cytokines secrete and lead. C57BL/6 mice are characterized by the development of a high level of TH1 cells and a high TH1 response [35]. Another immune system, the humoral immune response, this is mainly driven by an antibody-mediated immune response guided by TH2, which in C57BL/6 is predominantly TH1-responsive and weaker than the cell-mediated responses.
The results of research conducted by the Korean Food and Drug Administration for the localization of experimental animal resources produced a Korean local C57BL/6 stock, identified as C57BL/6NKorl, after inbreeding over 20 generations. C57BL/6NKorl was initially identified as C57BL/6J. However, that strain was confirmed as C57BL/6N after it was shown that an Nnt gene deletion did not occur, indicating no genetic differentiation from other cultivars. However, four single nucleotide polymorphisms (SNPs) proved C57BL/6NKorl to be a unique substrain. In 2015, the C57BL/6NKorl code was registered and certified by the Laboratory Animals Association (ILAR) under the National Institutes of Health, which manages the world's laboratory animal producer codes (Figure 2) [36].
Current inbred strains of mice are essential animal for laboratory research and such studies make clear contributions to many research fields. C57BL/6 mice is an inbred mouse strain originally developed for the study of antitumor activity and immunology, and is increasingly being used as an basic background strain. It is known that cell-mediated immunity and NK cell activity are of relatively high importance in determining immunological characteristics. In 2015, C57BL/6NKorl was established as a stock for N. The established C57BL/6NKorl is an independent stock showing genetic differences, based on SNP analyses, from other C57BL/6N. Studies into various characteristics of C57BL/6NKorl mice are expected to contribute to the use of this locally developed experimental animals and enhance the reliability and reproducibility of animal experiments.
Acknowledgments
This project was supported by a grant of BIOREIN (Laboratory Animal Bio Resources Initiative) from Ministry of Food and Drug Safety in 2016.
References
1. Festing MF. Properties of inbred strains and outbred stocks, with special reference to toxicity testing. J Toxicol Environ Health. 1979; 5(1):53–68. PMID: 423306.

2. WRIGHT S. The genetics of vital characters of the guinea pig. J Cell Comp Physiol. 1960; 56(Suppl 1):123–151.

3. Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat Genet. 2005; 37(11):1175–1180. PMID: 16254563.

4. Barros SF, Friedlanskaia I, Petricevich VL, Kipnis TL. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediators Inflamm. 1998; 7(5):339–346. PMID: 9883969.
5. Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl). 1997; 132(2):107–124. PMID: 9266608.

6. Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM Jr. Metabolic responses to leptin in obese db/db mice are strain dependent. Am J Physiol Regul Integr Comp Physiol. 2001; 281(1):R115–R132. PMID: 11404285.

7. Kile BT, Mason-Garrison CL, Justice MJ. Sex and strain-related differences in the peripheral blood cell values of inbred mouse strains. Mamm Genome. 2003; 14(1):81–85. PMID: 12532271.

8. Madiehe AM, Hebert S, Mitchell TD, Harris RB. Strain-dependent stimulation of growth in leptin-treated obese db/db mice. Endocrinology. 2002; 143(10):3875–3883. PMID: 12239099.
9. Nishina PM, Wang J, Toyofuku W, Kuypers FA, Ishida BY, Paigen B. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids. 1993; 28(7):599–605. PMID: 8355588.

10. Opsahl ML, McClenaghan M, Springbett A, Reid S, Lathe R, Colman A, Whitelaw CB. Multiple effects of genetic background on variegated transgene expression in mice. Genetics. 2002; 160(3):1107–1112. PMID: 11901126.

11. Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003; 52(8):1958–1966. PMID: 12882911.

12. Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol. 2012; 49(1):32–43. PMID: 22135019.
13. van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006; 5(2):139–149. PMID: 16507005.

14. Woodworth CD, Michael E, Smith L, Vijayachandra K, Glick A, Hennings H, Yuspa SH. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 2004; 25(9):1771–1778. PMID: 15105299.

15. Green MC, Grueneberg H, Hertwig P, Heston WE, Lyon MF, Medvedev NN, Snell GD, Staats J. A revision of the standardized genetic nomenclature for mice. J Hered. 1963; 54:159–162. PMID: 14057864.
16. Silver LM. Mouse genetics: concepts and applications. New York: Oxford University Press;1995. p. 3–31.
17. Dux A, Mhlbock O, Bailey DW. Genetic analyses of differences in incidence of mammary tumors and reticulum cell neoplasms with the use of recombinant inbred lines of mice. J Natl Cancer Inst. 1978; 61(4):1125–1129. PMID: 212568.
18. Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE. Mouse Genome Database Group. The mouse genome database (MGD): new features facilitating a model system. Nucleic Acids Res. 2007; 35(Database issue):D630–D637. PMID: 17135206.

19. Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, Yoshiki A. Genetic differences among C57BL/6 substrains. Exp Anim. 2009; 58(2):141–149. PMID: 19448337.

20. Zurita E, Chagoyen M, Cantero M, Alonso R, Gonzlez-Neira A, Lpez-Jimnez A, Lpez-Moreno JA, Landel CP, Bentez J, Pazos F, Montoliu L. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011; 20(3):481–489. PMID: 20506040.

21. Derhovanessian E, Solana R, Larbi A, Pawelec G. Immunity, ageing and cancer. Immun Ageing. 2008; 5:11. PMID: 18816370.

22. McVicar DW, Winkler-Pickett R, Taylor LS, Makrigiannis A, Bennett M, Anderson SK, Ortaldo JR. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J Immunol. 2002; 169(4):1721–1728. PMID: 12165492.

23. Malhotra A, Shanker A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy. 2011; 3(10):1143–1166. PMID: 21995569.

24. Mayer A, Lilly F, Duran-Reynals ML. Genetically dominant resistance in mice to 3-methylcholanthrene-induced lymphoma. Proc Natl Acad Sci U S A. 1980; 77(5):2960–2963. PMID: 6930679.

25. Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008; 27(45):5932–5943. PMID: 18836474.

26. Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980; 65(5):929–935. PMID: 6933263.
27. Corbett AJ, Coudert JD, Forbes CA, Scalzo AA. Functional consequences of natural sequence variation of murine cytomegalovirus m157 for Ly49 receptor specificity and NK cell activation. J Immunol. 2011; 186(3):1713–1722. PMID: 21187440.

28. Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001; 2(10):951–956. PMID: 11550009.

29. Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol. 2011; 11(5):307–317. PMID: 21475308.

30. Chen J, Flurkey K, Harrison DE. A reduced peripheral blood CD4(+) lymphocyte proportion is a consistent ageing phenotype. Mech Ageing Dev. 2002; 123(2-3):145–153. PMID: 11718808.

31. Chen J, Harrison DE. Quantitative trait loci regulating relative lymphocyte proportions in mouse peripheral blood. Blood. 2002; 99(2):561–566. PMID: 11781239.

32. Glineur S, Antoine-Moussiaux N, Michaux C, Desmecht D. Immune depression of the SJL/J mouse, a radioresistant and immunologically atypical inbred strain. Immunobiology. 2011; 216(1-2):213–217. PMID: 20965099.

33. Karamitros D, Kotantaki P, Lygerou Z, Kioussis D, Taraviras S. T cell proliferation and homeostasis: an emerging role for the cell cycle inhibitor geminin. Crit Rev Immunol. 2011; 31(3):209–231. PMID: 21740351.

34. Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 2011; 12(6):467–471. PMID: 21739668.

35. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000; 164(12):6166–6173. PMID: 10843666.

36. Ministry of Food and Drug Safety. Establishement for the stock of the domestic laboratory animals. Annu Rep 2015.
Figure 1
History of C57BL/6 substrain. C.C. Little established C57BL in 1921. C57BL/6 was isolated in 1937 and maintained in Jackson Laboratories (6J). C57BL/6N was isolated from Jackson Laboratories mice in 1951 and maintained by the NIH. Subsequently, the C57BL/6N strain moved to commercial companies, such as Charles River Laboratory, Harlan Sprague Dawley and Taconic Farms [20].
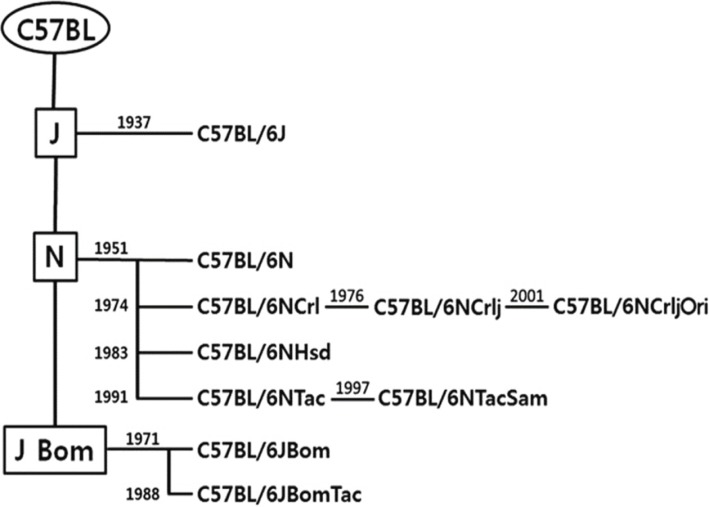
Figure 2
PCA (Principal component analysis) analysis was performed based on the results of 100 SNPs of 48 mice (12 per group). Use HelixTree SVS8 software for PCA analysis. Input the sample-specific genotype as an input file and calculate the vector that best separates the principal component. The most significant Eisen values 1 and 2 are plotted as PC1 and PC2, respectively. PCA analysis showed that C57BL/6NTac and C57BL/6NKorl form independent clusters, but C57BL/6NCrl and C57BL/6NHsd have the same genetic characteristics. Even in the same sample group, C57BL/6NKorl and C57BL/6NHsd have only the same genotype and Spot is not distinguished. C57BL/6NCrl and C57BL/6NTac have some genetic differences in the sample group [36].
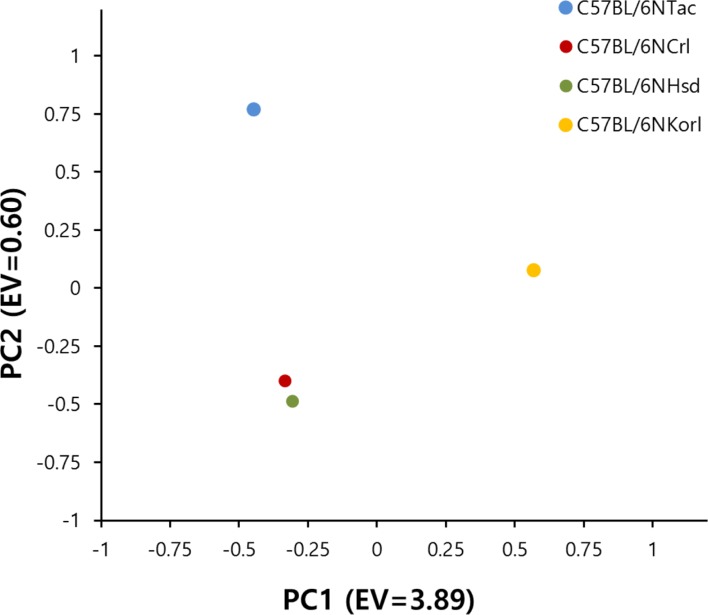
Table 1
Major biomedical fields with the application of C57BL/6 mice
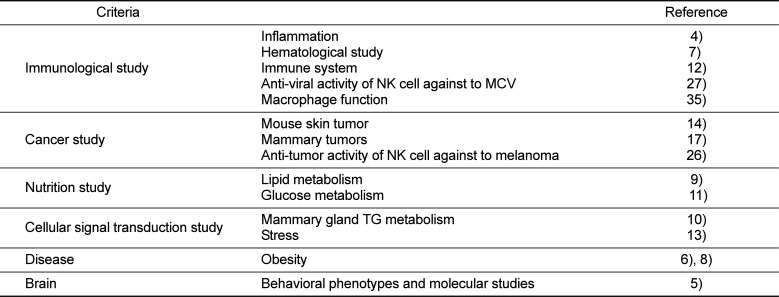
| Criteria | Reference | |
|---|---|---|
| Immunological study | Inflammation | 4) |
| Hematological study | 7) | |
| Immune system | 12) | |
| Anti-viral activity of NK cell against to MCV | 27) | |
| Macrophage function | 35) | |
| Cancer study | Mouse skin tumor | 14) |
| Mammary tumors | 17) | |
| Anti-tumor activity of NK cell against to melanoma | 26) | |
| Nutrition study | Lipid metabolism | 9) |
| Glucose metabolism | 11) | |
| Cellular signal transduction study | Mammary gland TG metabolism | 10) |
| Stress | 13) | |
| Disease | Obesity | 6), 8) |
| Brain | Behavioral phenotypes and molecular studies | 5) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download