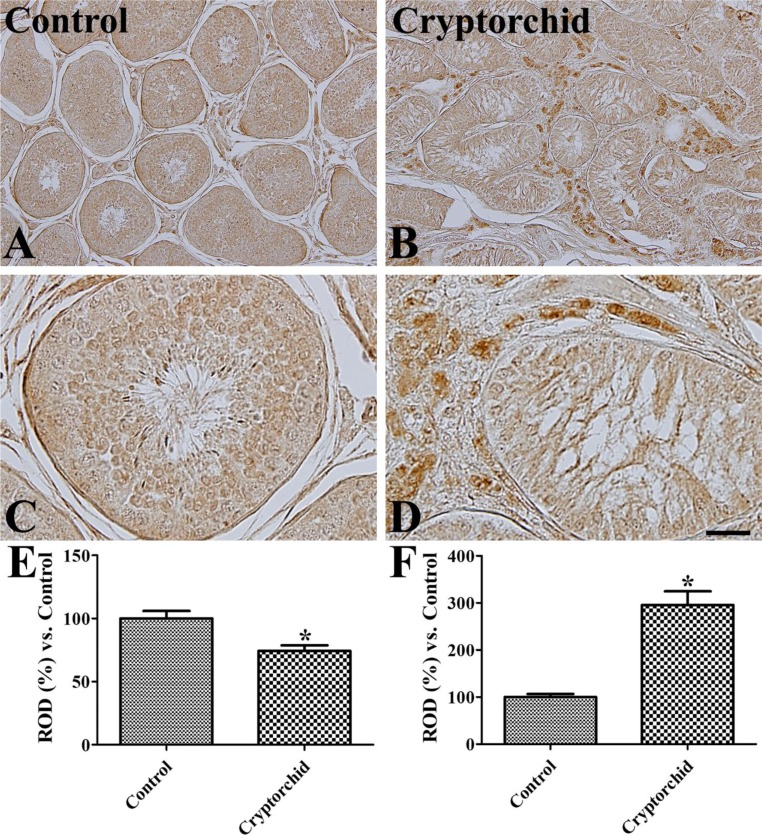
 | Figure 2Immunohistochemistry for GLUT3 in the control (scrotal, A and C) and cryptorchid (abdominal, B and D) testes. GLUT3 immunoreactivity is observed in mature spermatocytes and spermatids of the control testis whilst in the cryptorchid testis, GLUT3 immunoreactivity is only in the Leydig cells. Scale bar=100 µm (A and B), 25 µm (C and D). Relative optical density (ROD) of GLUT3 immunoreactivity in tubular (E) and extra-tubular (interstitial, F) regions per section is expressed as a percentage of the control group (10 sections, *P<0.05, which was significantly different from the control group). All data are represented as the mean±SE. |
Abstract
Glucose is essential for testicular function; the uptake of carbohydrate-derived glucose by cells is mediated by glucose transporters (GLUTs). In the present study, we investigated the activity of GLUT1 and GLUT3, the two main isoforms of GLUTs found in testes, in the left scrotal and right abdominal testes of a German Shepherd dog. Immunohistochemical analysis showed that GLUT1 immunoreactivity was absent in the scrotal and abdominal testes. In contrast, weak to moderate GLUT3 immunoreactivity was observed in mature spermatocytes as well as spermatids in the scrotal testis. In the abdominal testis, relatively strong GLUT3 immunoreactivity was detected in Leydig cells only and was absent in mature spermatocytes and spermatids. GLUT3 immunoreactivity was significantly decreased in the tubular region of abdominal testis and significantly increased in the extra-tubular (interstitial) region of abdominal testis compared to observations in the each region of scrotal testis, respectively. These results suggest that GLUT3 is the major glucose transporter in the testes and that abdominal testes may increase the uptake of glucose into interstitial areas, leading to an increased risk of developing cancer.
Go to : 
Testes have one of the highest rates of organ glucose uptake in order to maintain constant spermatogenesis [1], despite the sugar only existing at low levels in the seminiferous tubular fluid [2]. Uptake of glucose is controlled by specific glucose transporters (GLUTs) [3], which are reliant on its affinity to glucose in various organs. GLUT1, GLUT3, and GLUT8 are the main isoforms found in testes [4]. However, the expression of GLUTs is species-dependent. For example, GLUT1 expression has been observed in rat testes, GLUT2 in both mouse and rat testes, and GLUT3 in mouse, rat, and human testes [5]. In contrast, GLUT8 is primarily expressed in the acrosomal system including spermatids and spermatozoa [5]. This suggests that GLUT8 is linked to the energy supply of spermatozoa, and in the transportation of sugars during the capacitation and fertilization processes.
Cryptorchidism is a common clinical anomaly of the male genitalia and is one of the strongest risk factors for infertility and testicular cancer [6]. Several lines of evidence demonstrate that untreated cryptorchidism increases the risk of neoplasms including Sertoli cell tumors and seminomas [78]. In our previous study, we demonstrated morphological evidence for the proliferating properties of Sertoli cells in the abdominal testis, but not in the scrotal testis [9]. Previous research has reported that human testicular cancers show elevated GLUT3 mRNA levels in the tissue, indicating that GLUT3 may play a pivotal role in the uptake of glucose into cells [1011].
However, few studies have investigated the expression of GLUT1 and GLUT3 in the scrotal and abdominal testes in dogs, although the localization of GLUT1 and GLUT3 is quite different between the two species. In addition, we excluded to observe the localization of GLUT8 in the testes because we did not observe any mature spermatocytes and spermatids in the abdominal testis (Moon et al., 2014). Therefore, to further understand GLUT expression, we investigated the localization of GLUT1 and GLUT3 immunoreactivity in the scrotal and cryptorchid testes of a dog.
An 18-month-old German Shepherd (Canis lupus familiaris) with unilateral cryptorchidism was referred to the Seoul National University Veterinary Teaching Hospital, South Korea for elective orchidectomy, as explained in a previous study [9]. Upon examination, the left testis was present within the scrotum; however, the right testis was not palpable in the scrotum or inguinal area. Laparotomy revealed the cryptorchid testis in the right abdominal region. Both testes were surgically removed.
For histological analysis, both testes were fixed in neutral buffered formalin for two days and dehydrated with graded concentrations of alcohol before being embedded in paraffin. Paraffin-embedded tissues were sectioned into 3-µm coronal sections using a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and were mounted onto silane-coated slides (Muto Pure Chemicals Co., Ltd, Tokyo, Japan).
To ensure that the immunohistochemical data were comparable between the control (scrotal) and cryptorchid (abdominal) testis, the sections were processed under the same conditions. Sections were hydrated and treated with 0.3% hydrogen peroxide (H2O2) in phosphate-buffered saline (PBS) for 30 min. For antigen retrieval, sections were placed in 400-mL jars filled with citrate buffer (pH 6.0) and heated in a 2100-Retriever (Prestige Medical, Lancashire, UK). Following antigen retrieval, slides were cooled at 25℃ and were then washed in PBS. After washing, sections were incubated in 10% normal goat serum in PBS for 30 min. Sections were then incubated with a rabbit anti-GLUT1 (diluted 1:200, Abcam, Cambridge, UK), or a rabbit anti-GLUT3 antibody (diluted 1:50, SantaCruz Biotechnology, SantaCruz, CA, USA) for 48 h at 4℃. Following incubation, sections were exposed to biotinylated goat anti-rabbit IgG (diluted 1:200, Vector Laboratories, Inc., Burlingame, CA, USA), and streptavidin peroxidase complex (diluted 1:200, Vector Laboratories). Finally, sections were visualized with 3,3-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, USA) in 0.1 M Tris-HCl buffer (pH 7.4). Sections were mounted in Canada Balsam (Kanto Chemical, Tokyo, Japan) following dehydration.
Analysis of the regions of interest in the testes (tubular and extra-tubular area) was performed using an image analysis system. Images were calibrated into an array of 512×512 pixels corresponding to a tissue area of 1200 µm×900 µm (200× primary magnification). Each pixel resolution was 256 gray levels. The intensity of GLUT3 immunoreactivity was evaluated by relative optical density (ROD), which was obtained after transformation of the mean gray level using the following formula: ROD=log (256/mean gray level). The ROD of background was determined in the unlabeled tissue and this value was subtracted for correction. This approach yielded high ROD values in the presence of preserved structures and low values after structural loss using NIH Image 1.59 software (National Institutes of Health, Bethesda, MD, USA). A ratio of the ROD was calibrated as a percentage compared to the control (scrotal testis group).
In the scrotal testis, GLUT1 immunoreactivity was not observed in the any part of the seminiferous tubules or interstitial tissues (Figure 1A). Similarly, in the abdominal testis, GLUT1 immunoreactivity was not detected (Figure 1B).
Weak to moderate GLUT3 immunoreactivity was detected in spermatocytes and spermatids in the scrotal testis (Figure 2A, 2C). However, in the abdominal testis, GLUT3 immunoreactivity was not observed in these cells whilst strong immunoreactivity was observed in interstitial cells of the abdominal testis and weak immunoreactivity was found in the tubular structure (Figure 2B, 2D). Overall, GLUT3 immunoreactivity was significantly decreased in the tubular structures of abodominal testis compared to in the scrotal testis, while in the interstitial cells, GLUT3 immunoreactivity was significantly increased in the abdominal testis compared to that in the scrotal testis (Figure 2E).
Glucose is an essential source of energy for testicular development and function as well as for ongoing sperm cell development and quality [1213]. In the present study, we observed the immunoreactivity of two major GLUTs, GLUT1 and GLUT3, and compared their expression in the excised scrotal and abdominal testes in a single dog. We did not observe any GLUT1 immunoreactivity in the scrotal testis in the dog. This result is consistent with a previous study, which showed that GLUT1 expression was not observed in the testes of mice and humans [514]. Burant and Davidson [15] observed low but detectable levels of GLUT1 expression in human testes using western blot analysis. In the rat testes, GLUT1 immunoreactivity was observed only in differentiating spermatocytes in the type 1 stage, and not in mature spermatozoa [516]. Supporting these findings, Burant and Davidson [15] observed strong GLUT1 expression in rat testes using western blot analysis. In the present study, we did not observe expression of GLUT1 in the abdominal testis. This result suggests that GLUT1 has little or no impact on testicular function and cryptorchidism.
We also reported GLUT3 immunoreactivity in mature spermatocytes and spermatids in a canine scrotal testis. The distribution pattern of GLUT3 is different from the species used in the studies [5151718]. GLUT3 immunoreactivity is found only in the mature cells adjacent to the lumen of the seminiferous tubule as well as mature sperms in human [15], mouse [17], and rat [18] testes. However, Kokk et al. [14] reported GLUT3 expression in Sertoli cells, peritubular myoid cells, early spermatocytes, macrophage-like interstitial cells, and cells in the small vessel walls in human testes. In rat testes, GLUT3 immunoreactivity is found in all areas of the seminiferous epithelium as well as in mature sperms found in the lumen of the seminiferous tubule [15]. This discrepancy may be due to the antibody used in this study.
In the present study, we also observed GLUT3 immunoreactivity in Leydig cells, but not in mature spermatocytes and spermatids of the abdominal testis. The reduction of GLUT3 in the mature spermatocytes and spermatids may be associated with impaired spermatogenesis in the abdominal testis [9] because GLUT3 expression in these mature cells facilitates the uptake of glucose for successful fertilization [1920]. There has been conflicting evidence that GLUT3 expression is significantly decreased at 10, 20, and 30 days post-orchidectomy [21]. However, in a human study, the fluorodeoxyglucose uptake shows that GLUT3 expression remains within normal limits in the cryptorchid testis [22]. In this study, we observed strong GLUT3 immunoreactivity in the Leydig cells of an abdominal canine testis and overall slightly decreased GLUT3 immunoreactivity compared to observations in the scrotal testis. The strong expression of GLUT3 in the Leydig cells may be associated with cell hyperplasia and subsequent increase in the utilization of GLUT3 in the abdominal testis, as previous evidence suggests that uncorrected cryptorchidism can lead to Leydig cell hyperplasia in adults [23].
In conclusion, the expression patterns of GLUT1 and GLUT3 in dog testes are similar to that in humans. Our results support previous evidence that GLUT3 is the major GLUT isoform in testicular cells. Additionally, observed increases in GLUT3 in Leydig cells may be related to the hyperplasia in abdominal testis.
Acknowledgments
This result was supported by BK21 PLUS Program for Creative Veterinary Science Research, Research Institute for Veterinary Science and College of Veterinary Medicine, Seoul National University.
Go to : 
Notes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
Go to : 
References
1. Zysk JR, Bushway AA, Whistler RL, Carlton WW. Temporary sterility produced in male mice by 5-thio-D-glucose. J Reprod Fertil. 1975; 45(1):69–72. PMID: 1195257.
2. Robinson R, Fritz IB. Metabolism of glucose by Sertoli cells in culture. Biol Reprod. 1981; 24(5):1032–1041. PMID: 6268203.

3. Alves MG, Dias TR, Silva BM, Oliveira PF. Metabolic cooperation in testis as a pharmacological target: from disease to contraception. Curr Mol Pharmacol. 2014; 7(2):83–95. PMID: 25620223.

4. Oliveira PF, Alves MG, Rato L, Laurentino S, Silva J, Sá R, Barros A, Sousa M, Carvalho RA, Cavaco JE, Socorro S. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim Biophys Acta. 2012; 1820(2):84–89. PMID: 22146232.

5. Kokk K, Veräjänkorva E, Wu XK, Tapfer H, Põldoja E, Pöllänen P. Immunohistochemical detection of glucose transporters class I subfamily in the mouse, rat and human testis. Medicina (Kaunas). 2004; 40(2):156–160.
6. Hutson JM, Balic A, Nation T, Southwell B. Cryptorchidism. Semin Pediatr Surg. 2010; 19(3):215–224. PMID: 20610195.

7. Hayes HM Jr, Wilson GP, Pendergrass TW, Cox VS. Canine cryptorchism and subsequent testicular neoplasia: case-control study with epidemiologic update. Teratology. 1985; 32(1):51–56. PMID: 2863879.

8. Reif JS, Brodey RS. The relationship between cryptorchidism and canine testicular neoplasia. J Am Vet Med Assoc. 1969; 155(12):2005–2010. PMID: 4391618.
9. Moon JH, Yoo DY, Jo YK, Kim GA, Jung HY, Choi JH, Hwang IK, Jang G. Unilateral cryptorchidism induces morphological changes of testes and hyperplasia of Sertoli cells in a dog. Lab Anim Res. 2014; 30(4):185–189. PMID: 25628730.
10. Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Immunohistochemical detection of Glut3 in human tumors and normal tissues. Anticancer Res. 1997; 17(4A):2747–2750. PMID: 9252709.
11. Howitt BE, Brooks JD, Jones S, Higgins JP. Identification and characterization of 2 testicular germ cell markers, Glut3 and CyclinA2. Appl Immunohistochem Mol Morphol. 2013; 21(5):401–407. PMID: 23343953.

12. Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001; 22(4):680–695. PMID: 11451366.
13. Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl. 2007; 65:309–325. PMID: 17644971.
14. Kokk K, Veräjänkorva E, Laato M, Wu XK, Tapfer H, Pöllänen P. Expression of insulin receptor substrates 1-3, glucose transporters GLUT-1-4, signal regulatory protein 1alpha, phosphatidylinositol 3-kinase and protein kinase B at the protein level in the human testis. Anat Sci Int. 2005; 80(2):91–96. PMID: 15960314.

15. Burant CF, Davidson NO. GLUT3 glucose transporter isoform in rat testis: localization, effect of diabetes mellitus, and comparison to human testis. Am J Physiol. 1994; 267(6 Pt 2):R1488–R1495. PMID: 7810757.

16. Ibberson M, Riederer BM, Uldry M, Guhl B, Roth JR, Thorens B. Immunolocalization of GLUTX1 in the Testis and to Specific Brain Areas and Vasopressin-Containing Neurons. Endocrinology. 2002; 143(1):276–284. PMID: 11751619.
17. Kishimoto A, Ishiguro-Oonuma T, Takahashi R, Maekawa M, Toshimori K, Watanabe M, Iwanaga T. Immunohistochemical localization of GLUT3, MCT1, and MCT2 in the testes of mice and rats: the use of different energy sources in spermatogenesis. Biomed Res. 2015; 36(4):225–234. PMID: 26299481.
18. Rauch MC, Ocampo ME, Bohle J, Amthauer R, Yáñez AJ, Rodríguez-Gil JE, Slebe JC, Reyes JG, Concha II. Hexose transporters GLUT1 and GLUT3 are colocalized with hexokinase I in caveolae microdomains of rat spermatogenic cells. J Cell Physiol. 2006; 207(2):397–406. PMID: 16419038.

19. Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008; 295(2):E242–E253. PMID: 18577699.

20. Urner F, Sakkas D. A possible role for the pentose phosphate pathway of spermatozoa in gamete fusion in the mouse. Biol Reprod. 1999; 60(3):733–739. PMID: 10026124.
21. Farooqui SM, Al-Bagdadi F, O'Donnell JM, Stout R. Degenerative changes in spermatogonia are associated with loss of glucose transporter (Glut 3) in abdominal testis of surgically induced unilateral cryptorchidism in rats. Biochem Biophys Res Commun. 1997; 236(2):407–412. PMID: 9240450.

22. Kara PO, Kaya B, Gedik GK, Sari O, Varoglu E. Undescended testis in inguinal canal detected incidentally on fluorodeoxyglucose PET/CT imaging. Urology. 2012; 79(3):e29–e30. PMID: 22245311.

23. Gotoh M, Miyake K, Mitsuya H. Leydig cell hyperplasia in cryptorchid patients: quantitative evaluation of Leydig cells in undescended and contralateral scrotal testes. Urol Res. 1984; 12(3):159–164. PMID: 6148797.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download