1. Alwan A, Armstrong T, Bettcher D, Branca F, Chisholm D, Ezzati M, Garfield R, MacLean D, Mathers C, Mendis S, Poznyak V, Riley L, Tang KC, Wild C. Global Status Report on Noncommunicable Diseases 2010. World Health Organization;2011.
2. Lissin LW, Cooke JP. Phytoestrogens and cardiovascular health. J Am Coll Cardiol. 2000; 35(6):1403–1410. PMID:
10807439.
3. Park IS, Lee HW, Ryuk JA, Ko BS. Effects of an aqueous extract of dangguijagyagsan on serum lipid levels and blood flow improvement in ovariectomized rats. Evid Based Complement Alternat Med. 2014; 2014:497836. PMID:
25276217.

4. Peter FW, Franken RJ, Wang WZ, Anderson GL, Schuschke DA, O'Shaughnessy MM, Banis JC, Steinau HU, Barker JH. Effect of low dose aspirin on thrombus formation at arterial and venous microanastomoses and on the tissue microcirculation. Plast Reconstr Surg. 1997; 99(4):1112–1121. PMID:
9091911.

5. Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008; 451(7181):914–918. PMID:
18288180.

6. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58(11):1685–1693. PMID:
10571242.
7. Tachikawa E, Kudo K, Harada K, Kashimoto T, Miyate Y, Kakizaki A, Takahashi E. Effects of ginseng saponins on responses induced by various receptor stimuli. Eur J Pharmacol. 1999; 369(1):23–32. PMID:
10204677.

8. Nam KY. The comparative understanding between red ginseng and white ginsengs, processed ginsengs (Panax ginseng C.A. Meyer). J Ginseng Res. 2005; 29(1):1–18.
9. Seog H, Jung C, Kim Y, Park H. Phenolic acids and antioxidant activities of wild ginseng (Panax ginseng C.A. Meyer) leaves. Food Sci Biotechnol. 2005; 14(3):371–374.
10. Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001; 16(Suppl):S3–S5. PMID:
11748372.

11. Yamaguchi H, Matsuura H, Kasai R, Tanaka O, Satake M, Kohda H, Izumi H, Nuno M, Katsuki S, Isoda S, Shoji J, Gotoh K. Analysis of saponins of wild Panax ginseng. Chem Pharm Bull (Tokyo). 1988; 36(10):4177–4181. PMID:
3245991.

12. Paek KY, Murthy HN, Hahn EJ, Zhong JJ. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv Biochem Eng Biotechnol. 2009; 113:151–176. PMID:
19373448.

13. Sivakumar G. Bioreactor technology: a novel industrial tool for high-tech production of bioactive molecules and biopharmaceuticals from plant roots. Biotechnol J. 2006; 1(12):1419–1427. PMID:
17136730.

14. Sivakumar G, Yu K, Paek K. Production of biomass and ginsenosides from adventitious roots of Panax ginseng in bioreactor cultures. Eng Life Sci. 2005; 5(4):333–342.
15. Shah ZA, Gilani RA, Sharma P, Vohora SB. Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats. J Ethnopharmacol. 2005; 101(1):299–307. PMID:
15970412.

16. Han K, Shin IC, Choi KJ, Yun YP, Hong JT, Oh KW. Korea red ginseng water extract increases nitric oxide concentrations in exhaled breath. Nitric Oxide. 2005; 12(3):159–162. PMID:
15797844.

17. Lee E, Zhao H, Li D, Jeong C, Kim J, Kim Y. Effect of the MeOH extract of adventitious root culture of Panax ginseng on hyperlipidemic rat induced by high fat-rich diet. Korean J Pharmacogn. 2003; 34(2):179–184.
18. Ali MB, Hahn EJ, Paek K. Protective role of Panax ginseng extract on lipid peroxidation and antioxidant status in polyethylene glycol induced Spathiphyllum leaves. Biochem Eng J. 2006; 32(3):143–148.
19. Lim H, Kim Y, Lee D, Cho S, Cho M. The antifibrotic and antioxidant activities of hot water extract of adventitious root culture of Panax ginseng (ARCP). J Appl Biol Chem. 2007; 50(2):78–84.
20. Lee SO, Lee HJ, Yu MH, Im HG, Lee IS. Total polyphenol contents and antioxidant activities of methanol extracts from vegetables produced in Ulleung island. Korean J Food Sci Technol. 2005; 37(2):233–240.
21. Jin YR, Cho MR, Ryu CK, Chung JH, Yuk DY, Hong JT, Lee KS, Lee JJ, Lee MY, Lim Y, Yun YP. Antiplatelet activity of J78 (2-Chloro-3-[2'-bromo,4'-fluoro-phenyl]-amino-8-hydroxy-1,4-naphthoquinone), an antithrombotic agent, is mediated by thromboxane (TX) A2 receptor blockade with TXA2 synthase inhibition and suppression of cytosolic Ca
2+ mobilization. J Pharmacol Exp Ther. 2005; 312(1):214–219. PMID:
15328379.
22. Lee JJ, Yang H, Yoo YM, Hong SS, Lee D, Lee HJ, Lee HJ, Myung CS, Choi KC, Jeung EB. Morusinol extracted from Morus alba inhibits arterial thrombosis and modulates platelet activation for the treatment of cardiovascular disease. J Atheroscler Thromb. 2012; 19(6):516–522. PMID:
22472211.
23. Lee JJ, Jin YR, Yu JY, Munkhtsetseg T, Park ES, Lim Y, Kim TJ, Pyo MY, Hong JT, Yoo HS, Kim Y, Yun YP. Antithrombotic and antiplatelet activities of fenofibrate, a lipid-lowering drug. Atherosclerosis. 2009; 206(2):375–382. PMID:
19345949.

24. Born GV, Cross MJ. The aggregation of blood platelets. J Physiol. 1963; 168:178–195. PMID:
14056485.

25. Cowan DH, Robertson AL, Shook P, Giroski P. Platelet adherence to collagen: role of plasma, ADP, and divalent cations. Br J Haematol. 1981; 47(2):257–267. PMID:
6781525.

26. Morton LF, Peachey AR, Barnes MJ. Platelet-reactive sites in collagens type I and type III. Evidence for separate adhesion and aggregatory sites. Biochem J. 1989; 258(1):157–163. PMID:
2539100.

27. Poole AW, Watson SP. Regulation of cytosolic calcium by collagen in single human platelets. Br J Pharmacol. 1995; 115(1):101–106. PMID:
7647963.

28. Jin YR, Yu JY, Lee JJ, You SH, Chung JH, Noh JY, Im JH, Han XH, Kim TJ, Shin KS, Wee JJ, Yun YP. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007; 100(3):170–175. PMID:
17309520.

29. Jeon WK, Yoo BK, Kim YE, Park SO, Hahn EJ, Paek KY, Ko BS. Anti-platelet activity of tissue-cultured mountain ginseng adventitious roots in human whole blood. Food Sci Biotechnol. 2008; 17(6):1197–1202.
30. Ardlie NG, Garrett JJ, Bell LK. Collagen increases cytoplasmic free calcium in human platelets. Thromb Res. 1986; 42(2):115–124. PMID:
3715797.

31. Roberts DE, McNicol A, Bose R. Mechanism of collagen activation in human platelets. J Biol Chem. 2004; 279(19):19421–19430. PMID:
14981087.

32. Rittenhouse SE, Allen CL. Synergistic activation by collagen and 15-hydroxy-9 alpha,11 alpha-peroxidoprosta-5,13-dienoic acid (PGH2) of phosphatidylinositol metabolism and arachidonic acid release in human platelets. J Clin Invest. 1982; 70(6):1216–1224. PMID:
6816811.

33. Pollock WK, Rink TJ, Irvine RF. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986; 235(3):869–877. PMID:
3092807.

34. McNicol A, Nickolaychuk BR. Inhibition of collagen-induced platelet activation by arachidonyl trifluoromethyl ketone. Biochem Pharmacol. 1995; 50(11):1795–1802. PMID:
8615857.

35. Nakano T, Terawaki A, Arita H. Measurement of thromboxane A2-induced elevation of ionized calcium in collagen-stimulated platelets with the photoprotein, aequorin. J Biochem. 1986; 99(4):1285–1288. PMID:
3711064.
36. Feijge MA, van Pampus EC, Lacabaratz-Porret C, Hamulyak K, Levy-Toledano S, Enouf J, Heemskerk JW. Inter-individual variability in Ca
2+ signalling in platelets from healthy volunteers: effects of aspirin and relationship with expression of endomembrane Ca
2+-ATPases. Br J Haematol. 1998; 102(3):850–859. PMID:
9722316.
37. Shiraishi M, Ikeda M, Fujishiro T, Fukuyama K, Ito K. Characteristics of collagen-induced Ca2+ mobilization in bovine platelets. Cell Calcium. 2000; 27(1):53–60. PMID:
10726211.
38. Jang JY, Kim TS, Cai J, Kim J, Kim Y, Shin K, Kim KS, Lee SP, Kang MH, Choi EK, Rhee MH, Kim YB. Perilla oil improves blood flow through inhibition of platelet aggregation and thrombus formation. Lab Anim Res. 2014; 30(1):21–27. PMID:
24707301.

39. Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990; 60(4):269–280. PMID:
2087688.

40. Lockyer S, Kambayashi J. Demonstration of flow and platelet dependency in a ferric chloride-induced model of thrombosis. J Cardiovasc Pharmacol. 1999; 33(5):718–725. PMID:
10226858.

41. Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007; 21(3):131–142. PMID:
17208341.

42. Murthy HN, Dandin VS, Lee EJ, Paek KY. Efficacy of ginseng adventitious root extract on hyperglycemia in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2014; 153(3):917–921. PMID:
24709314.

43. Kim HJ, Chae IG, Lee SG, Jeong HJ, Lee EJ, Lee IS. Effects of fermented red ginseng extracts on hyperglycemia in streptozotocin-induced diabetic rats. J Ginseng Res. 2010; 34(2):104–112.

44. Wu J, Zhong JJ. Production of ginseng and its bioactive components in plant cell culture: current technological and applied aspects. J Biotechnol. 1999; 68(2-3):89–99. PMID:
10194851.

45. Matsuda H, Namba K, Fukuda S, Tani T, Kubo M. Pharmacological study on Panax ginseng C. A. Meyer. IV. Effects of red ginseng on experimental disseminated intravascular coagulation. (3). Effect of ginsenoside-Ro on the blood coagulative and fibrinolytic system. Chem Pharm Bull (Tokyo). 1986; 34(5):2100–2104. PMID:
3742652.
46. Matsuda H, Namba K, Fukuda S, Tani T, Kubo M. Pharmacological study on Panax ginseng C. A. Meyer. III. Effects of red ginseng on experimental disseminated intravascular coagulation. (2). Effects of ginsenosides on blood coagulative and fibrinolytic systems. Chem Pharm Bull (Tokyo). 1986; 34(3):1153–1157. PMID:
3731336.

47. Li CT, Wang HB, Xu BJ. A comparative study on anticoagulant activities of three Chinese herbal medicines from the genus Panax and anticoagulant activities of ginsenosides Rg1 and Rg2. Pharm Biol. 2013; 51(8):1077–1080. PMID:
23742679.
48. Lee SR, Park JH, Kim ND, Choi KJ. Inhibitory effects of ginsenoside Rg3 on platelet aggregation and its mechanism of action. Korean J Ginseng Sci. 1997; 21:132–140.
49. Yu KW, Gao W, Hahn EJ, Paek KY. Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng C.A. Meyer. Biochem Eng J. 2002; 11(2-3):211–215.
50. Kim YS, Hahn EJ, Murthy HN, Paek KY. Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol Lett. 2004; 26(21):1619–1622. PMID:
15604808.

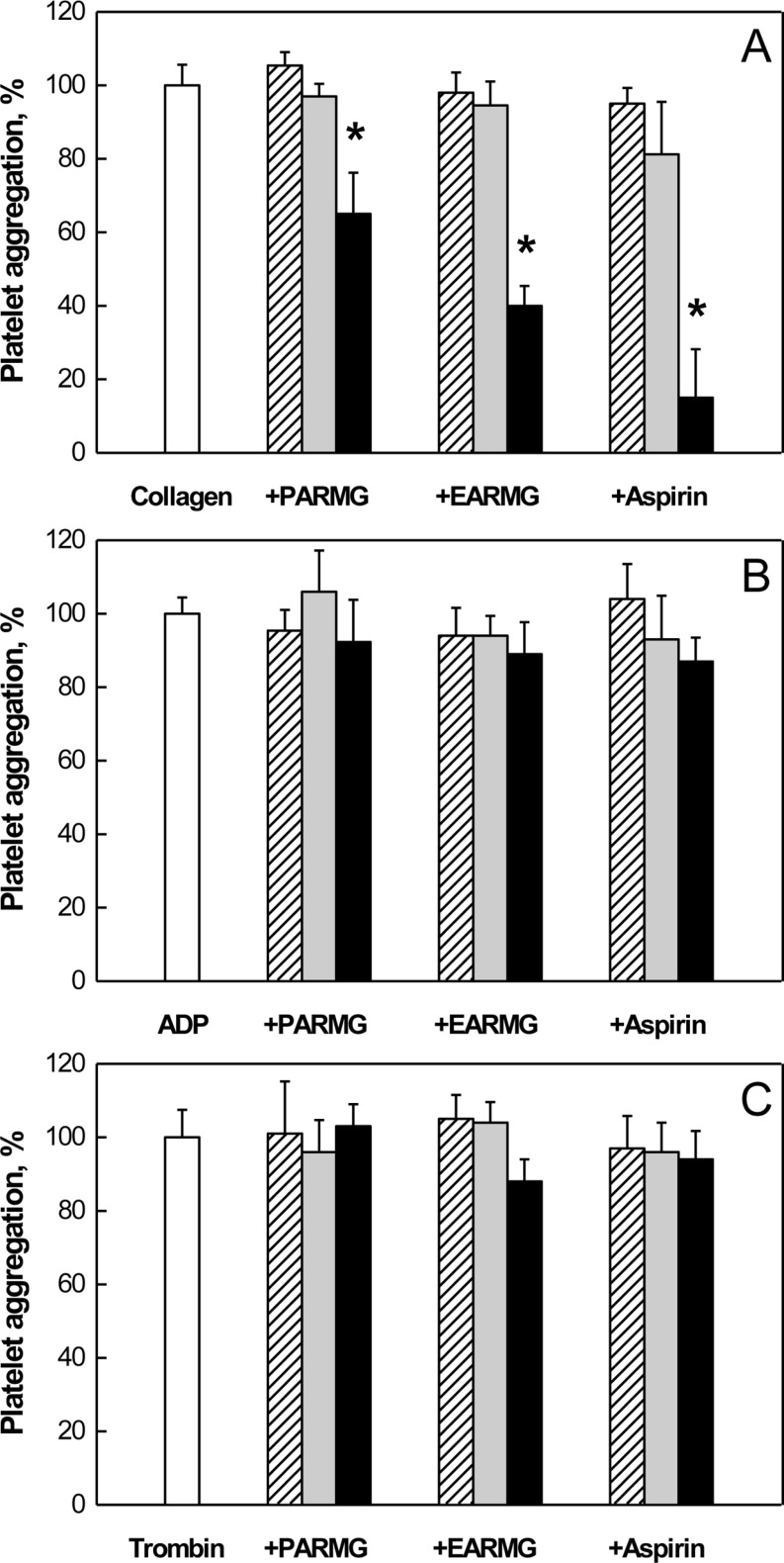
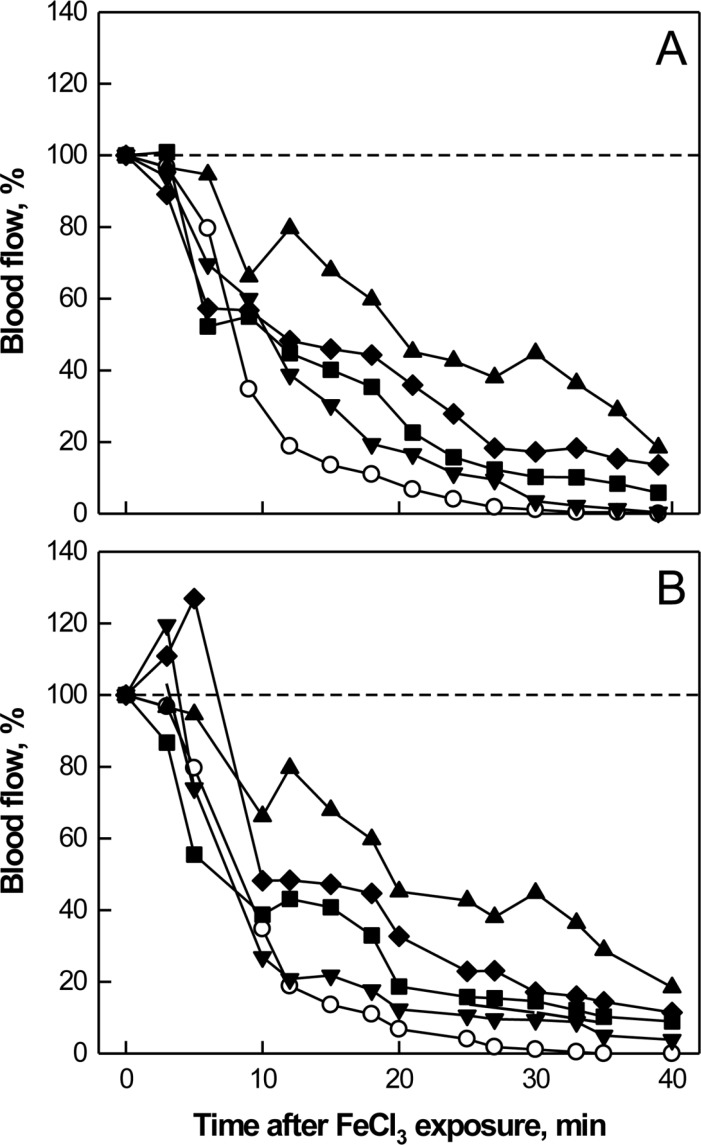
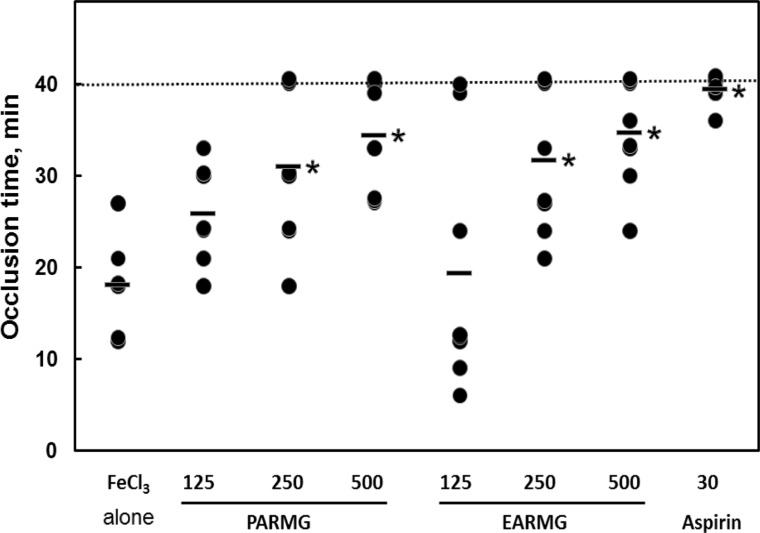
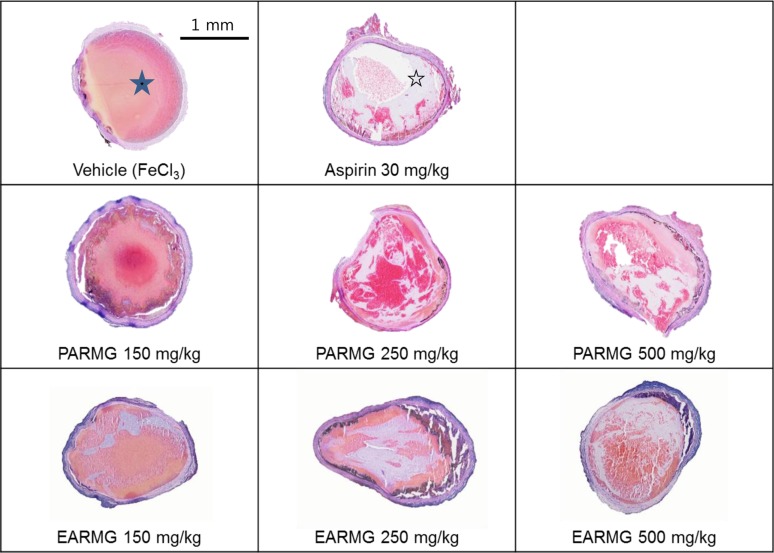




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download