Abstract
Helicobacter pylori colonizes the gastric mucosa of about half of the world's population, causing chronic gastritis and gastric cancer. An increasing emergence of antibiotic-resistant H. pylori arouses demand on alternative non-antibiotic-based therapies. In this study, we freshly prepared crude N-acetylneuraminic acid obtained from glycomacropeptide (G-NANA) of whey through a neuraminidase-mediated reaction and evaluated its antibacterial ability against H. pylori and H. felis. Overnight cultures of the H. pylori were diluted with fresh media and different concentrations (1-150 mg/mL) of crude G-NANA were added directly to the culture tube. Bacterial growth was evaluated by measuring the optical density of the culture medium and the number of viable bacteria was determined by a direct count of the colony forming units (CFU) on agar plates. For the in vivo study, mice were orally infected with 100 µL (5×108 cfu/mL) of H. felis four times at a day's interval, accompanied by a daily administration of crude G-NANA or vehicle. A day after the last infection, the mice were daily administered the crude G-NANA (0, 75, and 300 mg/mL) for 10 days and euthanized. Their stomachs were collected and bacterial colonization was determined by quantitative real-time PCR. Crude G-NANA inhibited H. pylori's growth and reduced the number of viable bacteria in a dose-dependent manner. Furthermore, crude G-NANA inhibited bacterial colonization in the mice. These results showed that crude G-NANA has antibacterial activity against Helicobacter and demonstrated its therapeutic potential for the prevention of chronic gastritis and gastric carcinogenesis induced by Helicobacter infection in humans.
Helicobacter pylori is the causative agent of chronic gastritis, peptic ulcer, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [12]. H. pylori colonizes the gastric mucosa of 80% and 40% of adults in developing and industrialized countries, respectively [3]. After entering the stomach, H. pylori penetrates and adheres to mucin and gastric epithelial cells through various adhesion molecules [4]. Over 90% of H. pylori are thought to be present in the mucous layers while the remainder colonize the gastric surface [5]. There are two major H. pylori virulence factors that influence the pathogenesis of H. pylori infections: cytotoxin-associated genes pathogenecity island (CagPAI) and vacuolating cytotoxin A (VacA) [67]. The CagPAI encodes components of a type IV secretion system (T4SS) that can inject the CagA protein and other factors into the host cells, inducing more severe inflammatory disease [6]. VacA damages gastric epithelial cells by forming pores in cell membranes [7]. Interferon (IFN)-γ-producing CD4+ T cells play a critical role in the control of H. pylori infections and in vaccine-induced protection [89]. However, these Th1 immune responses are known to be inhibited by transforming growth factors (TGF)-β produced by gastric epithelial cells and regulatory T (Treg) cells, which are induced by H. pylori on infecting the host [10]. Currently, the most effective therapeutic treatment is the combination of a proton pump inhibitor with antibiotics [11]. However, an increasing emergence of antibiotic-resistant H. pylori has led to eradication failures and a demand for alternative non-antibiotic therapies.
Sialic acids are a family of monosaccharides with a nine-carbon backbone and are present in the central nervous system and in body fluids including saliva, tears, and milk [12]. The predominant sialic acid found in mammals is N-acetylneuraminic acid (NANA or Neu5Ac) [12] and the highest concentration of sialic acid is found in the mammalian central nervous system, where it promotes brain development [13]. In addition, sialic acid has antimicrobial potential [14]. Pathogenic microorganisms including bacteria, viruses, and parasites attach to a host cell's surface carbohydrates during the initial stages of infection. As sialylated oligosaccharides in mucin and milk can be a decoy for binding of pathogens, sialylated oligosaccharides can inhibit infection by preventing the binding of pathogens [14]. In fact, sialylated oligosaccharides in human milk prevent the binding of pathogenic virus, bacteria, and toxins to target cells [141516].
Previous studies on the antimicrobial potential of sialic acid focused on its inhibition of the binding of pathogens. However, in this study, we prepared crude N-acetylneuraminic acid obtained from glycomacropeptide (G-NANA) and demonstrated its bactericidal activity against H. pylori. Furthermore, we examined the therapeutic potential of crude G-NANA against Helicobacter infection in a murine model.
Crude G-NANA was obtained from the glycomacropeptide (GMP) of whey through an enzymatic reaction with a neuraminidase isolated from Arthrobacter ureafaciens. Briefly, A. ureafaciens was grown in an M9 medium containing 10% GMP as a carbon source for 16 h at 30℃ with shaking at 150 rpm. The cultures were filter-pressed, and crude enzymes with over 20 kDa molecular weight were collected by ultra-filtration. Ultra-filtrated crude enzymes were reacted with GMP of whey at 55℃ for 5 h and heat-inactivated at 95℃ for 30 min. Concentrations of G-NANA (65 mg/g) in the enzymatic reaction was determined by quantitative high-performance liquid chromatography (HPLC) analysis.
H. pylori strains P1WT and SS1, and H. felis have been reported previously [1718]. H. pylori was routinely grown on Campylobacter agar plates or Brucella broth (BD, Sparks, NV, USA) containing 10% of FBS, 10 µg/ mL of vancomycin (Sigma-Aldrich, California, USA), 5 µg/mL of trimethoprim (Sigma), and 1 µg/mL of nystatin (Sigma) at 37℃ under microaerobic conditions using GasPak™ EZ Campy Container System (BD). H. felis was grown on Brucella broth containing 10% of FBS at 37℃ under microaerobic conditions using GasPak™ EZ Campy Container System (BD).
Overnight cultures of the bacteria were diluted with fresh broth media. Crude G-NANA was added at various concentrations (0-150 mg/mL) directly to the culture tubes and the turbidity was monitored at 12 and 24 h later by measuring the optical density at 600 nm. To determine the number of viable H. pylori P1WT and SS1 from the 24-h culture incubated with crude G-NANA, each culture was serially diluted and spread on agar plates. After 5-day incubation under microaerobic conditions, the colonies were counted.
Wild-type (WT) C57BL/6 mice were purchased from Koatech (Pyeongtaek, Gyounggido, Korea). The mice were divided into two groups. Group 1 mice were fasted for 12 h and orally infected with 100 µL (5×108 cfu/mL) of H. felis for four times at 2-day interval; they were administered crude G-NANA (75 or 300 mg/mL) daily until the last infection. Group 2 mice were fasted for 12 h and orally infected with 100 µL (5×108 cfu/mL) of H. felis for four times at 2-day interval. One day after the last infection, mice from both group 1 and 2 were daily administered the crude G-NANA (75 or 300 mg/mL) for 10 days and euthanized by cervical dislocation. Their stomachs were removed, opened longitudinally along the greater curvature, and washed with sterile PBS. The tissues were stored at −20℃ for DNA extraction to determine bacterial colonization. The animal studies were conducted under approved protocols by the Institutional Animal Care and Use Committee of Chonnam National University (Gwangju, Korea). [Approval No. CNU IACUC-YB-R-2015-58].
An equal size of small sections of the stomachs, including the fundus was prepared for DNA extraction. DNA was extracted using the Tissue DNA kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer's instructions and real-time PCR was performed using the Qiagen SYBR green PCR kit (QIAGEN GmbH, Hilden, Germany). The following primer sequences were used. FlaB forward: 5'-TTCGATTGGTCCTACAG GCTCAGA-3', reverse: 5'-TTCTTGTTGATGACATTG ACCAACGCA-3'; Gapdh forward: 5'-TCAAGAAGGT GGTGAAGCAGG-3', reverse: 5'-TATTATGGGGGTC TGGGATGG-3'. PCR amplification was performed using a two-step cycle of 95℃ for 15 s followed by 58℃ for 1 min for 40 cycles in a Roter-GeenQ Real-time PCR system (QIAGEN, GmbH, Hilden, Germany). Relative gene expression of H. felis FlaB was calculated with the 2−ΔΔCt method using Gapdh as the internal control [19]. Standards of relative gene expression were made by sequential 10-fold dilutions of known colony forming units (cfu) of H. felis in an uninfected stomach piece and used to estimate the cfu of H. felis in infected mice.
The differences in mean values among different groups were tested, and the values were expressed as mean ± SD. All of the statistical calculations were performed by one or two-way ANOVA with Bonferroni post-tests using GraphPad Prism version 5.00. Values of *P<0.05, **P<0.01, and ***P<0.001 were considered significant.
We first evaluated the effect of crude G-NANA on the growth of H. pylori. 2×107 cfu/mL of the H. pylori strains P1WT and SS1 were incubated with various concentrations (0, 3, 15, 30, 75 and 150 mg/mL) of crude G-NANA for 12 and 24 h and bacteria growth was determined by measuring the optical density of the cultures at a wavelength of 600 nm [20]. The growth of both H. pylori P1WT and SS1 was inhibited by crude GNANA at 30, 75, and 150 mg/mL (Figures 1A and B). This inhibitory effect increased in a crude G-NANA concentration-dependent manner from 30 to 150 mg/mL (Figures 1A and B). Next, we determined the number of viable bacteria from the 24 h culture of H. pylori incubated with crude G-NANA by counting colony forming units (CFU)/mL from serial tenfold dilutions of each culture. Crude G-NANA at the concentrations of 75 and 150 mg/mL drastically decreased CFU of both H. pylori strains P1WT and SS1 compared to that of the untreated bacterial cultures whereas 30 mg/mL of crude G-NANA slightly decreased the CFU of both H. pylori P1WT and SS1 cultures (Figure 2A and B). These results demonstrated that crude G-NANA has growth inhibition activity against the H. pylori strains P1WT and SS1.
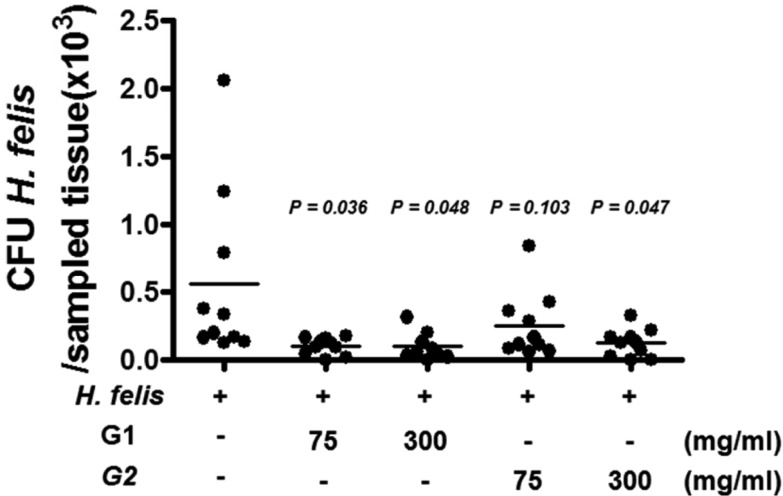
To examine the in vivo effect of crude G-NANA in Helicobacter infections, we used an H. felis murine model [21]. Consistent with results on the growth of H. pylori, Crude G-NANA inhibited the in vitro growth of H. felis at concentrations of 15 mg/mL onwards (Figure 3). For in vivo experiment, as described in Materials and Methods, mice were divided into three groups; H. felis infected group, G-NANA treated group (from 1st day of infection) with H. felis infection (group 1), and G-NANA treated group (from the day after last day of infection) with H. felis infection (group 2). In group 1, both 75 and 300mg/mL crude G-NANA significantly reduced bacterial colonization in the stomach (Figure 4). In group 2, 300 mg/mL of crude G-NANA, but not 75 mg/mL, administration for 10 days after the last bacterial infection significantly reduced bacterial colonization in the stomach (Figure 4). Although 75 mg/mL of crude G-NANA inoculation from a day after the last infection for 10 days showed little effect on bacterial colonization, the same concentration of crude G-NANA administered from the first infection until the last experiment reduced bacterial colonization (Figure 4). These results indicate that crude G-NANA has an inhibitory effect on the gastric colonization of Helicobacter.
Although antibiotic-based therapies for H. pylori infections are available, the emergence of antibiotic-resistant bacteria coupled with the risk of re-infection and the high cost of antibiotic therapy make the bacterial eradication more difficult. Because of these challenges, many studies have focused on the development of effective vaccines and alternative treatments using non-antibiotic compounds. To develop a successful vaccine for inducing protective immunity against H. pylori, determining potent bacterial antigens, effective adjuvants and the best delivery route are critical. Due to its high expression in all H. pylori isolates, urease has been considered as a good candidate for vaccine antigens and has provided efficient protection against infections when used with other antigens [22]. Aluminum hydroxide is an approved adjuvant in clinical applications and has efficiently promoted antigen-specific humoral and cellular immune responses against intramuscularly delivered antigens in humans [23]. However, continued efforts to develop a safe and effective vaccination for humans are required.
There have been increasing studies on non-antibiotic-based therapies including plant extracts and probiotics as potential alternatives for the treatment of H. pylori infections. Green tea is one of the well-studied therapeutic candidates for H. pylori infection [2425]. Green tea showed antibacterial activity against H. pylori in vitro and decreased bacterial colonization and inflammation in animal models [2425]. In addition, catechins and polyphenols present in green tea appeared to be important for the inhibition of urease and the VacA toxin, respectively [2526]. Although other plants such as broccoli, garlic and wine have been known to have antibacterial activity against H. pylori in vitro, very few of them show in vivo antibacterial activity [272829]. Probiotics, which are live microorganism that provide health benefits when consumed, showed antibacterial effects against H. pylori in vitro and in vivo [303132]. Lactobacillus casei strain Shirota, L. acidophilus, L. rhamnosus, and Bacillus subtilis inhibited H. pylori infections in mouse models [303132]. The beneficial effects of probiotics on H. pylori infection included decreased gastric inflammation and reduced bacterial colonization in mice [33].
To our knowledge, this is the first report on the isolation of NANA from glycomacropeptides through a neuraminidase-mediated reaction. In addition, the discovery of its antibacterial activity against H. pylori is novel. Furthermore, crude G-NANA administration inhibited bacterial colonization in the stomach. Since sialic acid was shown to prevent the binding of H. pylori to various human gastric epithelial cells in vitro [34] and inhibit bacterial colonization in rhesus monkeys [35], the reduced bacterial colonization could be attributed to the crude G-NANA-induced inhibition of bacterial binding to gastric epithelium. However, the observation that crude G-NANA administration reduced bacterial colonization significantly (Figure 2) while sialic acid effectively controlled H. pylori infection in mice when used with antioxidants, but not alone [36], indicates that the reduced bacterial colonization induced by crude G-NANA is most likely a result of its bactericidal activity against H. pylori rather than its inhibition of the bacteria's binding.
In this study, we freshly prepared crude G-NANA that showed antibacterial activity against H. pylori in vitro and in vivo, demonstrating its therapeutic potential in H. pylori infection.
Acknowledgment
This work was supported by a grant (No. 112140032 SB010) from the Korea Institute of Planning and Evaluation for Technology in Food Agriculture, Forestry and Fisheries.
References
1. Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998; 114(6):1169–1179. PMID: 9609753.
2. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001; 345(11):784–789. PMID: 11556297.
3. Perez-Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004; 9(Suppl 1):1–6. PMID: 15347299.
4. Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993; 262(5141):1892–1895. PMID: 8018146.
5. Mobley HLT, Mendz MG, Hazell SL. Helicobacter; physiology and genetics. Washington, D.C: ASM press;2001. p. 381–417.
6. Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008; 10(8):1573–1581. PMID: 18410539.
7. Cover TL. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996; 20(2):241–246. PMID: 8733223.
8. Sayi A, Kohler E, Hitzler I, Arnold I, Schwendener R, Rehrauer H, Müller A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009; 182(11):7085–7101. PMID: 19454706.
9. Akhiani AA, Pappo J, Kabok Z, Schön K, Gao W, Franzén LE, Lycke N. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J Immunol. 2002; 169(12):6977–6984. PMID: 12471132.
10. Beswick EJ, Pinchuk IV, Earley RB, Schmitt DA, Reyes VE. Role of gastric epithelial cell-derived transforming growth factor beta in reduced CD4+ T cell proliferation and development of regulatory T cells during Helicobacter pylori infection. Infect Immun. 2011; 79(7):2737–2745. PMID: 21482686.
11. O'Connor A, Vaira D, Gisbert JP, O'Morain C. Treatment of Helicobacter pylori infection 2014. Helicobacter. 2014; 19:38–45. PMID: 25167944.
12. Wang B, Brand-Miller J. The role and potential of sialic acid in human nutrition. Eur J Clin Nutr. 2003; 57(11):1351–1369. PMID: 14576748.

13. Tram TH, Brand Miller JC, McNeil Y, McVeagh P. Sialic acid content of infant saliva: comparison of breast fed with formula fed infants. Arch Dis Child. 1997; 77(4):315–318. PMID: 9389234.

14. Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993; 3(2):97–130. PMID: 8490246.

15. Parkkinen J, Finne J, Achtman M, Väisänen V, Korhonen TK. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983; 111(2):456–461. PMID: 6340671.
16. Idota T, Kawakami H, Murakami Y, Sugawara M. Inhibition of cholera toxin by human milk fractions and sialyllactose. Biosci Biotechnol Biochem. 1995; 59(3):417–419. PMID: 7766178.

17. Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Mémet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004; 5(11):1166–1174. PMID: 15489856.
18. Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, Misselwitz R, Berger J, Sewald N, König W, Backert S. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007; 449(7164):862–866. PMID: 17943123.

19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4):402–408. PMID: 11846609.
20. Campbell J. High-throughput assessment of bacterial growth inhibition by optical density measurements. Curr Protoc Chem Biol. 2010; 2(4):195–208. PMID: 23839976.

21. Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996; 64(1):238–245. PMID: 8557346.

22. Flach CF, Svensson N, Blomquist M, Ekman A, Raghavan S, Holmgren J. A truncated form of HpaA is a promising antigen for use in a vaccine against Helicobacter pylori. Vaccine. 2011; 29(6):1235–1241. PMID: 21147129.
23. Malfertheiner P1, Schultze V, Rosenkranz B, Kaufmann SH, Ulrichs T, Novicki D, Norelli F, Contorni M, Peppoloni S, Berti D, Tornese D, Ganju J, Palla E, Rappuoli R, Scharschmidt BF, Del Giudice G. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology. 2008; 135(3):787–795. PMID: 18619971.
24. Stoicov C, Saffari R, Houghton J. Green tea inhibits Helicobacter growth in vivo and in vitro. Int J Antimicrob Agents. 2009; 33(5):473–478. PMID: 19157800.
25. Matsubara S, Shibata H, Ishikawa F, Yokokura T, Takahashi M, Sugimura T, Wakabayashi K. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun. 2003; 310(3):715–719. PMID: 14550260.
26. Tombola F, Campello S, De Luca L, Ruggiero P, Del Giudice G, Papini E, Zoratti M. Plant polyphenols inhibit VacA, a toxin secreted by the gastric pathogen Helicobacter pylori. FEBS Lett. 2003; 543(1-3):184–189. PMID: 12753930.
27. Sivam GP. Protection against Helicobacter pylori and other bacterial infections by garlic. J Nutr. 2001; 131(3s):1106S–1108S. PMID: 11238826.
28. Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002; 99(11):7610–7615. PMID: 12032331.
29. Mahady GB, Pendland SL, Chadwick LR. Resveratrol and red wine extracts inhibit the growth of CagA+ strains of Helicobacter pylori in vitro. Am J Gastroenterol. 2003; 98(6):1440–1441. PMID: 12818294.
30. Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G, Tsakalidou E, Mentis A. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol. 2004; 70(1):518–526. PMID: 14711683.
31. Lorca GL, Wadström T, Valdez GF, Ljungh A. Lactobacillus acidophilus autolysins inhibit Helicobacter pylori in vitro. Curr Microbiol. 2001; 42(1):39–44. PMID: 11116395.
32. Pinchuk IV, Bressollier P, Verneuil B, Fenet B, Sorokulova IB, Mégraud F, Urdaci MC. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob Agents Chemother. 2001; 45(11):3156–3161. PMID: 11600371.
33. Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004; 49(7-8):1095–1102. PMID: 15387328.

34. Simon PM, Goode PL, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun. 1997; 65(2):750–757. PMID: 9009338.
35. Mysore JV, Wigginton T, Simon PM, Zopf D, Heman-Ackah LM, Dubois A. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology. 1999; 117(6):1316–1325. PMID: 10579973.
36. Yang JC, Shun CT, Chien CT, Wang TH. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS cells and BALB/c mice. J Nutr. 2008; 138(11):2084–2090. PMID: 18936202.
Figure 1
Crude N-acetylneuraminic acid obtained from glycomacropeptide (G-NANA) inhibits the growth curve of H. pylori. H. pylori P1WT (A) and SS1 (B) were grown overnight in the culture medium. The bacteria culture was diluted with fresh broth media. Crude G-NANA was added at various concentrations (0-150 mg/mL) directly to the culture media and turbidity was monitored 12 and 24 h later by measuring optical density of the medium at 600 nm. Data are shown as mean ± SD from one experiment representative of more than three independent experiments (**p<0.01 and ***P<0.001).
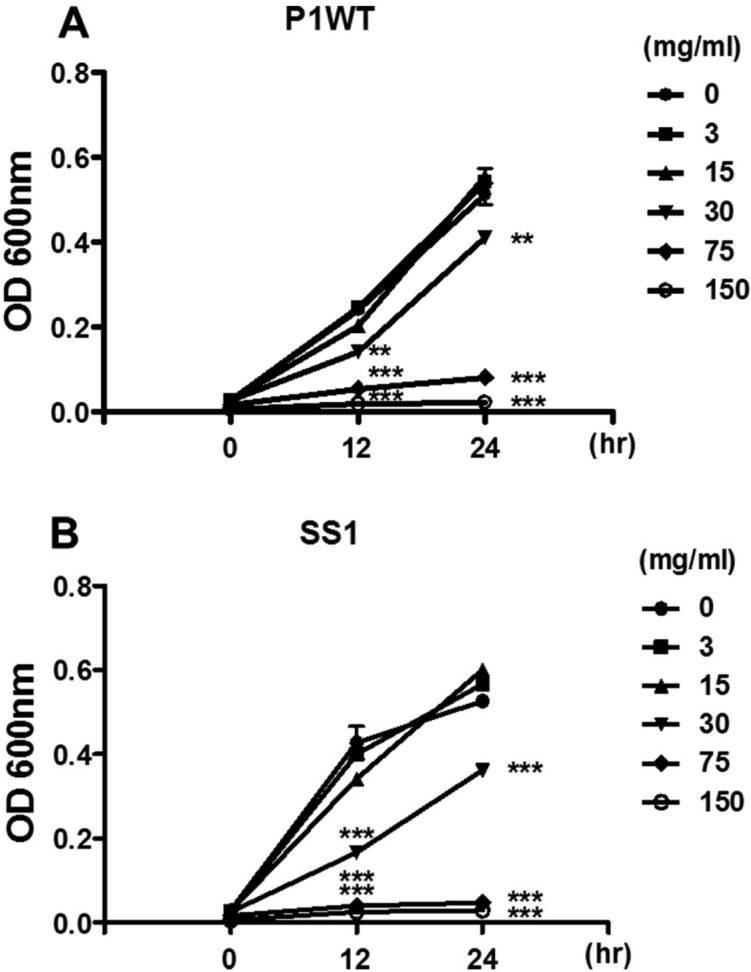
Figure 2
Crude N-acetylneuraminic acid obtained from glycomacropeptide (G-NANA) reduced the number of viable H. pylori. H. pylori P1WT (A) and SS1 (B) were prepared as described in Fig. 1. Twenty-four hours after bacterial culture with crude G-NANA at various concentrations (0-150 mg/mL), each culture was serially diluted and spread on agar plates. The colony was counted after 5 days incubation under microaerobic conditions. Data are shown as mean ± SD of triplicate samples from one experiment representative of three independent experiments (**P<0.01 and ***P<0.001).
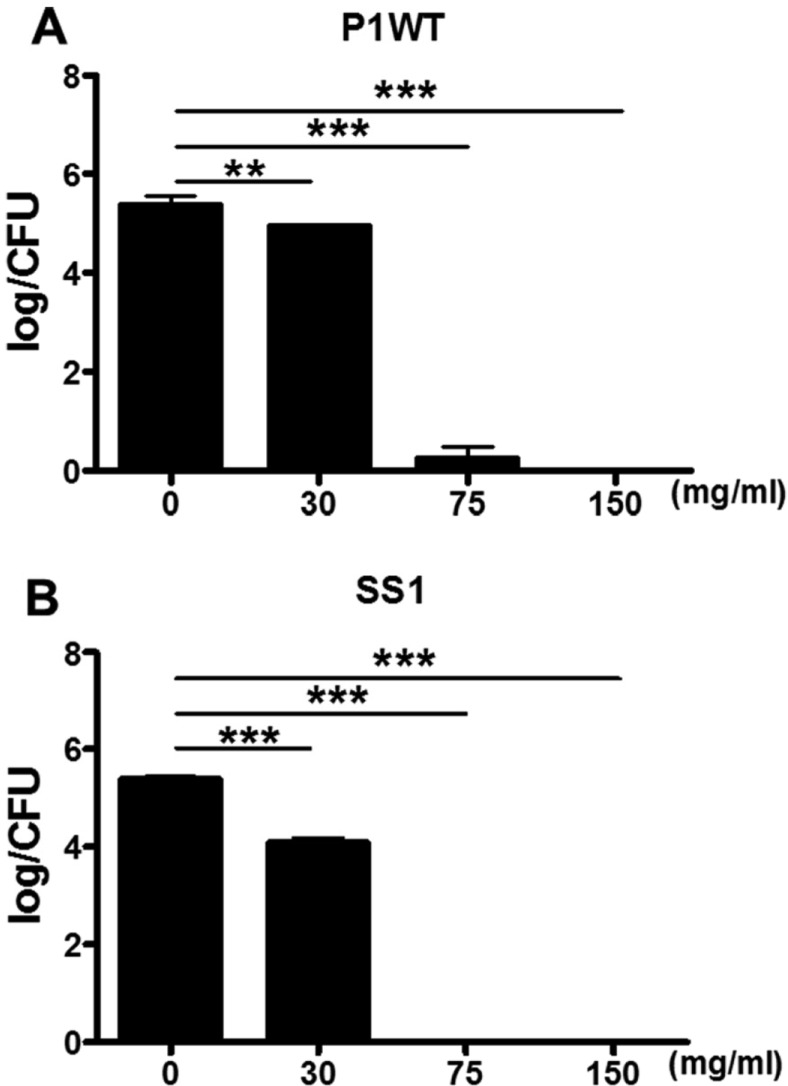
Figure 3
Crude N-acetylneuraminic acid obtained from glycomacropeptide (G-NANA) inhibits the growth curve of H. felis. H. felis was grown overnight in the culture medium. The bacteria culture was diluted with fresh broth media. Crude GNANA was added at various concentrations (0-150 mg/mL) directly to the culture media and the turbidity was monitored 48 h later by measuring the optical density of the medium at 600 nm. Data are shown as mean ± SD from one experiment representative of more than three independent experiments (**P<0.01 and ***P<0.001).
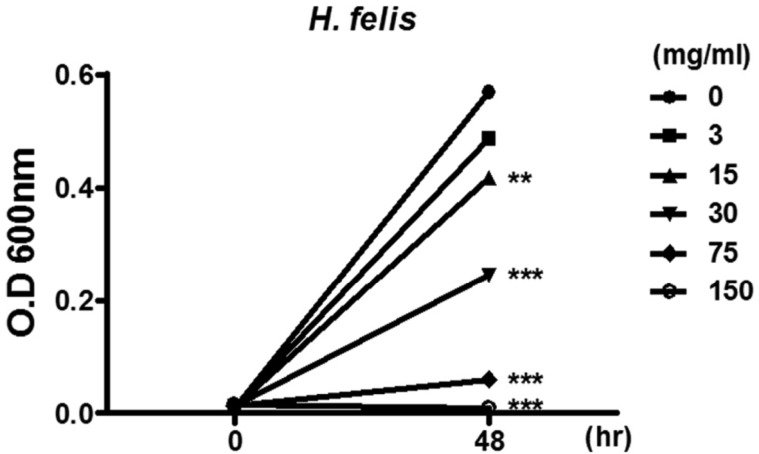
Figure 4
Crude N-acetylneuraminic acid obtained from glycomacropeptide (G-NANA) inhibits gastric colonization of H. felis. Mice were divided into two groups: Group 1 (G1); mice were fasted for 12 h and orally infected with 100 µL (5×108 cfu/ mL) of H. felis four times at 1-day interval with daily administration of crude G-NANA (75 or 300 mg/mL) until last infection, Group 2 (G2); mice were fasted for 12 h and orally infected with 100 µL (5×108 cfu/mL) of H. felis four times at 1- day interval. One day after last infection, mice from both group 1 and 2 were daily administered the crude G-NANA (75 or 300 mg/mL) for 10 days and euthanized by CO2 inhalation. The stomachs were collected and bacterial colonization was determined by quantitative real-time PCR as described in materials and methods. Data are shown as mean ± SD (n =6) from one experiment representative of two independent experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download