This article has been
cited by other articles in ScienceCentral.
Abstract
The antibacterial activities of the essential oil of Eucalyptus globulus (EOEG) was determined against 7 fish pathogenic bacteria (Edwardsiella tarda, Streptococcus iniae, S. parauberis, Lactococcus garviae, Vibrio harveyi, V. ichthyoenteri and Photobacterium damselae) obtained from farmed olive flounder. The inhibitory activity was evaluated by three methods: Disc diffusion method, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). According to the disc diffusion test, as the concentration of EOEG (5-40 µg) rises, the inhibitory zone increases in size. Compared with amoxicillin, tetracycline and chloramphenicol, EOEG showed similar antibacterial activity. The MIC of EOEG ranged from 7.8 to 125 mg/mL and MBC values ranged from 62 to 250 mg/mL. These results show that EOEG has antimicrobial activity against all seven bacteria, but there was no marked difference between each genus. From these results, it is suggested that EOEG can be used as an antimicrobial agent against fish bacterial diseases in the fish industry.
Go to :

Keywords: Eucalyptus globulus, antibacterial activity, fish pathogenic bacteria
Industrial aquaculture in Korea has made rapid developments within a short period, and the output of fish farms has grown under the influence of increased consumption caused by an improved standard of living [
1]. However, susceptibility to infection has increased due to overcrowded rearing, excessive feeding for mass production, deterioration of the fishing ground by long-term intensive culture, and an increase in recessive breeds; and this has caused huge economic losses in recent years. As a result, industrial development has been accompanied by some practices potentially damaging to human and animal health that include passing large amounts of veterinary drugs into the environment [
23]. Accordingly, various studies have been made to search for antimicrobial agents from natural materials like
Rhus javanica extracts and bee venom for application to aquaculture [
45]. Such natural substances are safer for the human body than antibiotics and chemicals and are not as concerned with resistant bacteria [
6].
The essential oil constituents of the genus Eucalyptus (
Myrtaceae) have been well characterized [
7]. Eucalyptus species produce numerous volatile compounds in large amounts, especially isoprenoids, which accumulate in glands abundantly distributed throughout the leaf parenchyma and bark [
8].
The chemical compositions of the leaf oils of various Eucalyptus species have been reported [
91011]. The major component was 1,8-cineole, but a main contributor for the bioactivity was assumed to be terpineol, which showed eight-fold higher antimicrobial activity than 1,8-cineole against
Staphylococcus aureus. Moreover, 1,8-cineole has not been reported as an active principle in other eucalyptus oils [
12]. Studies on eucalyptus extracts as natural antibiotics on virulent bacteria in humans have continued [
1516], but to date none have been performed on fish pathogenic bacteria. Therefore, this study was carried out for the purpose of demonstrating the antibacterial activities of the essential oil of
Eucalyptus globulus (hereafter referred to as EOEG) on the seven pathogenic bacteria (
Edwardsiella tarda, Streptococcus iniae, S. parauberis, Lactococcus garviae, Vibrio harveyi, V. ichthyoenteri and
Photobacterium damselae) that pose the largest threats to flatfish farming in Korea.
Materials and Methods
Bacteria and EOEG
As experimental strains, four gram-negative strains and three gram-positive strains isolated from Korean cultured olive flounder (Paralichthys olivaceus) were used. The four gram-negative strains were E. tarda FP 5060 (2005), V. harveyi FP 8370 (2009), V. ichthyoenteri FP 4004 (2004), P. damselae FP 4101 (2004) and the 3 gram-positive strains were L. garviae FP 5245 (2005), S. iniae FP 5228 (2005), and S. parauberis FP 3287 (2007). These bacteria were received from the National Fisheries Research and Development Institute (Busan, Korea). Our EOEG was purchased from Aromaland Inc., Santa fe, NM, USA who had purified it through steam distillation from the leaves of wild Eucalyptus globulus grown in Australia.
Disc Diffusion Method
Prior to testing antimicrobial effects, a fresh colony cultured for 24 hours at 25℃ in tryptic soy agar (TSA) (MBcell Ltd., Seoul, Korea) was used to suspend the organism in 0.85% sterilized saline solution with a concentration of MacFaland No. 0.5 (1-5×106 CFU/mL). Each 100 µL of the test organism's suspension water was distributed into Muller-Hinton agar (MHA) (MBcell Ltd., Seoul, Korea) in a 4-5 mm thick petri dish (87×15 mm) and was well coated with a sterilized cotton swab to dry moisture. EOEG (5, 10, 20, 40 µg) was dried on sterilized paper discs (ϕ10 mm) (Advantec Toyo Kaisha Ltd., Tokyo, Japan) to absorb 20 µL of moisture, and each disc was stuck to a MHA containing test organisms to be cultured in a culture chamber. The medium coated with test bacteria was cultured for 24 hours at 25℃ to determine the antimicrobial effects. Each test was repeated three times.
Several antibiotic discs were used as controls against EOEG. A single antibiotic from several types was selected for the test: amoxicillin (30 µg/disc) for β-lactam type, tetracycline (30 µg/disc) for tetracycline type, erythromycin (15 µg/disc) for macrolide type, nalidixic acid (30 µg/disc) for quinolone type, and chloramphenicol (30 µg/disc) for phenicol type. All antibiotics were purchased from MBcell Ltd., Seoul, Korea. The five antibiotics' antimicrobial effects on fish pathogenic bacteria were tested using the same method as with EOEG. Each antibiotic disc absorbed 20ß° of moisture with the concentration adjusted such that the amount of the antibiotic contained in commercially available disks was used.
MIC and MBC
The MIC test was performed using the broth dilution method. Target organisms were cultured in TSA one night at 25℃ then diluted to a concentration of MacFarland No.0.5 (1-5×106 CFU/mL). EOEG was gradually diluted in Muller-Hinton Broth until it reached 488-500,000 µg/mL, and target organisms were injected. The MIC was then measured 48 hours after the initial injection. Each test was repeated three times.
For measurement of the MBC, a culture solution with a concentration greater than the MIC was smeared in MHA agar medium, inoculated with strains and cultivated for 24 hours at 25℃. The MBC was determined as the concentration of the maximum dilution factor which killed 99.9% of the initial number of inoculated strain.
Go to :

Results
Disc Diffusion Method
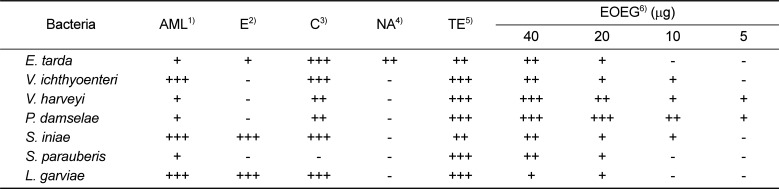
We measured the diameter of the inhibition zone formed around the paper disk containing a concentration of the EOEG from low (5 µg) to high concentrations (40 µg) for each tested pathogen. The inhibition zone size was proportional to the concentration of the essential oil, and, in the case of high concentration (40 µg), significant antibacterial activity similar to antibiotics used in the control group was shown (
Table 1). The average of three measurements was used.
Table 1
Antimicrobial activity of EOEG and antibiotics by the disk diffusion test
|
Bacteria |
AML1)
|
E2)
|
C3)
|
NA4)
|
TE5)
|
EOEG6) (µg) |
|
40 |
20 |
10 |
5 |
|
E. tarda
|
+ |
+ |
+++ |
++ |
++ |
++ |
+ |
- |
- |
|
V. ichthyoenteri
|
+++ |
- |
+++ |
- |
+++ |
++ |
+ |
+ |
- |
|
V. harveyi
|
+ |
- |
++ |
- |
+++ |
+++ |
++ |
+ |
+ |
|
P. damselae
|
+ |
- |
++ |
- |
+++ |
+++ |
+++ |
++ |
+ |
|
S. iniae
|
+++ |
+++ |
+++ |
- |
++ |
++ |
+ |
+ |
- |
|
S. parauberis
|
+ |
- |
- |
- |
+++ |
++ |
+ |
- |
- |
|
L. garviae
|
+++ |
+++ |
+++ |
- |
+++ |
+ |
+ |
- |
- |

In the case of the commercial antibiotics implemented as controls, amoxicillin, chloramphenicol and tetracycline showed susceptibility in every tested strain, but nalidixic acid and erythromycin did not show susceptibility in 6 tested strains (
Table 1).
MIC and MBC
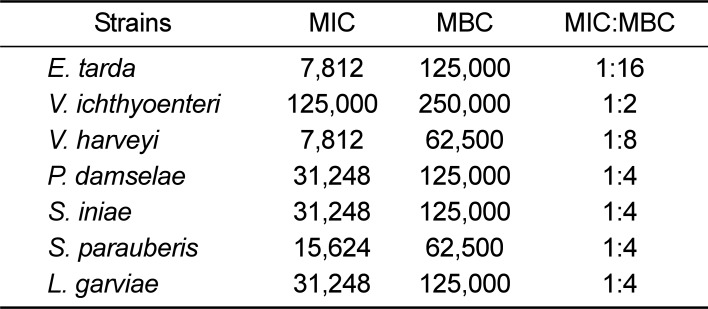
For 7 fish pathogenic bacteria, EOEG was two-fold diluted in sterile distilled water to 488-250,000 µg/mL in stages, and the MIC and the MBC were measured. Against all strains except
V. Ichthyoenteri, a significant effect was shown with an MIC ranging from 7,812-31,248 µg/mL.
V. Ichthyoenteri, however, showed a much greater MIC of 125,000 µg/mL. EOEG showed the lowest value against
E. tarda and
V. harveyi, 7,812 µg/mL. The MIC values for the remaining pathogens are shown in
Table 2. The values shown were averaged over three measurements.
Table 2
MIC and MBC values (µg mL-1) of EOEG against 7 different bacteria
|
Strains |
MIC |
MBC |
MIC:MBC |
|
E. tarda
|
7,812 |
125,000 |
1:16 |
|
V. ichthyoenteri
|
125,000 |
250,000 |
1:2 |
|
V. harveyi
|
7,812 |
62,500 |
1:8 |
|
P. damselae
|
31,248 |
125,000 |
1:4 |
|
S. iniae
|
31,248 |
125,000 |
1:4 |
|
S. parauberis
|
15,624 |
62,500 |
1:4 |
|
L. garviae
|
31,248 |
125,000 |
1:4 |

With respect to the MBC values, they ranged from being identical to the MIC or much greater as shown in
Table 2. In the case of
V. harveyi and
S. parauberis, the MBC was measured as 62,500 µg/mL, and for
E. tarda, P. damselae, S. iniae and
L. garviae it was measured as 125,000 µg/mL. In the case of
V. Ichthyoenteri, the MBC was measured as 250,000 µg/mL, twice the MIC value.
Go to :

Discussion
Although antibiotics have been used as an effective way to control infectious bacterial disease for a long time, the use of many antibiotics has been prohibited in a number of countries [
13]. Accordingly, research on natural antibiotics that can replace conventional antibiotics has emerged. It has been demonstrated that the essential oil extracted from
E. globulus is effective as an insect repellent, medicine for respiratory diseases, antiinflammatory drug, antibacterial agent against
S. aureus and
Escherichia coli, and antitumor agent in rats [
1415161718].
In the disc diffusion test, 5 antibiotics and EOEG at the concentrations 5, 10, 20, and 40 µg were absorbed into discs and introduced onto cultures of 7 fish pathogenic bacteria. In every fish pathogenic bacteria, the inhibition zone increased in proportion to the EOEG concentration. Significantly, at the highest concentration (40 µg), EOEG showed effective antibacterial activity similar to each control antibiotic except nalidixic acid. In measurement of the MIC, E. tarda and V. harveyi showed relatively higher susceptibility compared with other strains, and four strains showed relative resistance. Finally, V. ichthyoenteri showed the highest resistance to EOEG.
According to the results, EOEG has antibacterial activity against 7 strains of fish pathogenic bacteria when present at a quantity (40 µg) similar to that of antibiotics on the market through the disc diffusion test. Through the MIC and MBC tests, we further found evidence that EOEG has antibacterial activity against fish pathogenic bacteria.
South Korea still contains only a small number of farmers planting eucalyptus because it possesses neither a subtropical nor tropical climate. Moreover, research on the effects of Eucalyptus is also lacking compared to other countries. However, the antimicrobial efficacy of EOEG was found against seven bacteria which are problems in the flat fish aquaculture industry in Korea. EOEG showed antibacterial activity even on strains that have resistance to erythromycin and nalidixic acid, both of which have been used in Korea. This demonstrates that EOEG could replace some preexisting antibiotics. In addition, our experiment focused only on the essential oil extracted from the leaves of E. globulus, which is only one of over 900 varieties of eucalyptus. If experiments on different varieties of eucalyptus and essential oil extraction method are carried out, they could be highly useful for aquatic animal medicines. However, further studies on safety and toxicity are necessary to confirm its therapeutic efficacy in vivo against these pathogenic bacteria.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download