Abstract
Nonhuman primates are increasingly used in biomedical research since they are highly homologous to humans compared to other rodent animals. However, there is limited reliable reference data of the clinical pathology parameters in cynomolgus monkeys, and in particular, only some coagulation and urinalysis parameters have been reported. Here, we reported the reference data of clinical chemical, hematological, blood coagulation, and urinalysis parameters in cynomolgus monkeys. The role of sex differences was analyzed and several parameters (including hematocrit, hemoglobin, red blood cell, blood urea nitrogen, total bilirubin, alkaline phosphatase, creatinine kinase, gamma-glutamyl tranferase, and lactate dehydrogenase) significantly differed between male and female subjects. In addition, compared to previous study results, lactate dehydrogenase, creatinine kinase, and aspartate aminotransferase showed significant variation. Interstudy differences could be affected by several factors, including age, sex, geographic origin, presence/absence of anesthetics, fasting state, and the analytical methods used. Therefore, it is important to deliberate with the overall reference indices. In conclusion, the current study provides a comprehensive and updated reference data of the clinical pathology parameters in cynomolgus monkeys and provides improved assessment criteria for evaluating preclinical studies or biomedical research.
Cynomolgus monkeys (Macaca fascicularis) are one of the most widely employed nonhuman primate species in drug development, toxicology, and biomedical research. Cynomolgus monkeys appear to originate from tropical insular Southeast Asia and are commonly observed near seashore, swamps, and river banks [1]. The typical life span of a cynomolgus monkey ranges from 25-30 years and males and females attain sexual maturity at six and four years of age, respectively [2]. Cynomolgus monkeys are anatomically and physiologically homologous to humans compared to other animal models (e.g., rats, dogs). Furthermore, they share many other characteristics with humans. They are omnivorous, reflecting the diverse habitats they live in and they are susceptible to age-related pathologies commonly observed in humans. Because of the increased use of cynomolgus monkey in nonclinical studies, it is necessary to establish the clinical pathology parameters in this monkey species to monitor their health and pathological status.
Clinical pathology testing including clinical chemistry, hematology, blood coagulation, and urinalysis is a standard element of nonclinical toxicology studies. These test results provide information regarding organ systems, metabolic function, and pathophysiologic response. Reference values are important in clinical and preclinical studies to evaluates health condition indices and improve the accuracy of drug safety studies. In addition, these indices are valuable for assessing possible effects in early investigational or discovery-type studies that lack control animals or baseline data [3]. In order to build an integrated, high-quality foundation for future cynomolgus monkey-based research, comprehensive and accurate reference values of clinical pathology parameters are required. The values obtained in this study will be used as baseline data for clinical chemistry, hematologic, blood coagulation, and urinalysis parameters in healthy cynomolgus monkeys.
Pretest results of clinical pathology parameters were examined in 18 preclinical toxicology studies carried out at Korea Institute of Toxicology (KIT) between 2011 and 2014. The study population comprised 113 cynomolgus monkeys (76 males and 37 females; age: 24-60 months; body weights: 2-4 kg) obtained from NafoVanny (Tam Phuoc Hamlet, Bien Hoa City, Dong Nai Province, Vietnam). All imported monkeys were quarantined at the nonhuman primate facility at Korea Institute of Toxicology (KIT) for at least 30 days. Throughout the study, the animal room environment was controlled (temperature maintained at 20-29℃, humidity of 40-70%, approximately 12-hour light/12-hour dark cycle (08:00 to 20:00) with 150-300 lux, ventilation 10-20 times/hour, and negative pressure), and monkeys were housed individually in a stainless-steel cage (543W × 715L × 818H mm). The groups received filtered, ultraviolet light-irradiated municipal tap water ad libitum and were fed approximately 60 g of food (Certified Primate Diet #5048, PMI nutrition International, Inc.) twice daily. The animals were managed at KIT, an accredited animal facility, complying with the AAALAC International Animal Care Policies. The Animal Care and Use Committee of the KIT reviewed and approved all the study protocols.
The parameters' abbreviations, units, and analysis methods are presented in Table 1. All animals were fasted overnight and blood samples were collected from the cephalic vein of non-anesthetized monkey for analysis of hematologic, coagulation, and serum chemistry parameters; if blood cannot be obtained from the cephalic vein, the saphenous or femoral vein was used. Blood samples were collected in tubes containing K2-EDTA for hematologic analysis. Hematological parameters were analyzed using an Advia 2120i hematology analyzer (Siemens, USA).
For serum chemistry analysis, blood samples were collected in tubes and placed at room temperature for at least 90 min prior to centrifugation (approximately 3000 rpm, 10 minutes, at room temperature). Serum chemistry parameters were measured using a Toshiba 200FR NEO chemistry analyzer (Toshiba Co., Japan).
In addition, blood samples were collected in tubes containing 3.2% sodium citrate and plasma samples were obtained after centrifugation (approximately 3000 rpm, 10 minutes, at room temperature) and analyzed for prothrombin (PT), activated partial thromboplastin time (APTT), and fibrinogen (FIB) using an ACL 9000 coagulation analyzer (Instrumental Laboratory, Italy).
Animals were fasted overnight prior to urine collection. Urine samples were collected with a clean cage pan on wet ice (first void in the morning of each collection day), and the total fresh urine volume (VOL) was recorded. Urine samples were collected in a urine specimen container and kept cool with wet ice before analysis. Urinalysis was performed using a COBAS U 411 urine analyzer (Roche, Germany) and Combur 10 TM urine stick (Roche, Germany) to determine the following parameters: color, specific gravity (SG), pH, urine total protein (UTP), urine glucose (UGLU), urine creatinine (UCRE), urine albumin (UALB), and urine protein to creatinine ratio (P/C). Urine potassium (U-K), urine chlorine (U-Cl), and urine sodium (U-Na) were measured using a Toshiba 200FR NEO chemistry analyzer (Toshiba Co., Japan).
Unpaired t-test was applied to detect significant differences between male and female subjects (Tables 2,3,4,5). The significance level was set to 0.05 (*), 0.01 (**), 0.001 (***), and 0.0001 (****). All data processing, data management, and statistical analysis were performed using Prism 6 for Windows (Version 6.0 5 (trial), GraphPad Software Inc.).
In this study, hematological and biochemical analyses of 113 cynomolgus monkeys (76 males and 37 females) were performed in order to establish baseline reference indices for the macaque species. Also, limited data is available on coagulation and urinalysis parameters with some updated data on clinical chemistry parameters (e.g., PL, LDL, HDL, CRP, and C3) in cynomolgus monkeys. These reference indices are essential in evaluating preclinical findings and selecting healthy subjects.
Although there are a few published studies reporting hematological and biochemical values in cynomolgus monkeys, these values may be influenced by various factors. Previous studies that have established reference values in cynomolgus monkeys with similar ages [145] are compared to the current study to identify inter-study variability.
First, in the case of hematologic parameters, there were marked similarities between our study and previous study results (Tables 2 and 4). This indicates that the hematology data of cynomolgus monkeys are consistent and are not affected by environmental variables; hence, these data could be used to interpret the health of the monkey regardless of the region.
Second, regarding clinical chemistry parameters, the values of aspartate transaminase (AST), alanine transaminase (ALT), LDH, CK, TCHO, CREA, and ALP observed in the present study were higher than those reported by Tadashi et al. (2005) [4]; further, LDH, CK, and AST values were higher than those reported by Schuurman and Smith (2005) [5] (Tables 3 and 5). Intra- and inter-study difference in nonhuman primates were known to more variable compare to rodent species. The variables that may account for inter-study variations in cynomolgus monkey include age, sex, geographic origin, presence/absence of anesthetics, fasting, and gravity [67891011]. In addition, analytical instruments, reagents, and methods are variables that could influence the values obtained for a particular parameter, particularly in serum chemistry analysis. These factors are considered by comparing our data to those of previous studies (analytical methods are shown in Table 1). The analytical instrument and methods (ALT, ALP, LDH, CREA, and Ca) employed by Tadashi et al. (2005) [4] were different to those employed in our study. Physiologic variance of the indices also affected inter-study variation. ALP is present in biliary and canalicular membranes, the kidney, intestine, and bone and age-related changes in osseous ALP were observed, reflecting bone growth in juvenile periods [1213]. LDH and CK were affected by skeletal muscle mass and there were many false positives for myotoxicity because of background injury associated with handling or restraint, especially with nonhuman primates since there were struggles, trauma, fever, and muscle puncture during sampling procedures [14]. Therefore, when applying these parameters, age, sex, body weight, and analytical methods should be considered to evaluate the data accurately.
Third, in the coagulation study, mean PT and APTT values in male and female monkeys were 9.76 s and 9.75 s and 19.06 s and 19.33 s, respectively. No significant differences in PT and APTT were observed between male and female juvenile cynomolgus monkeys (Tables 3 and 5). Our study showed a similar result as previous research. However, it appears that there are significant differences in PT and APTT values between juvenile cynomolgus and rhesus monkeys; hence, the type of subspecies monkey should be considered [15]. Last, regarding the urinalysis parameters, values for urine SG, pH, U-K, U-Cl, and U-Na were similar between male and female monkeys (Tables 2 and 4). There is limited urinalysis data from previous study of cynomolgus monkeys; the urinalysis data obtained from the present study will be valuable for future study of cynomolgus monkeys.
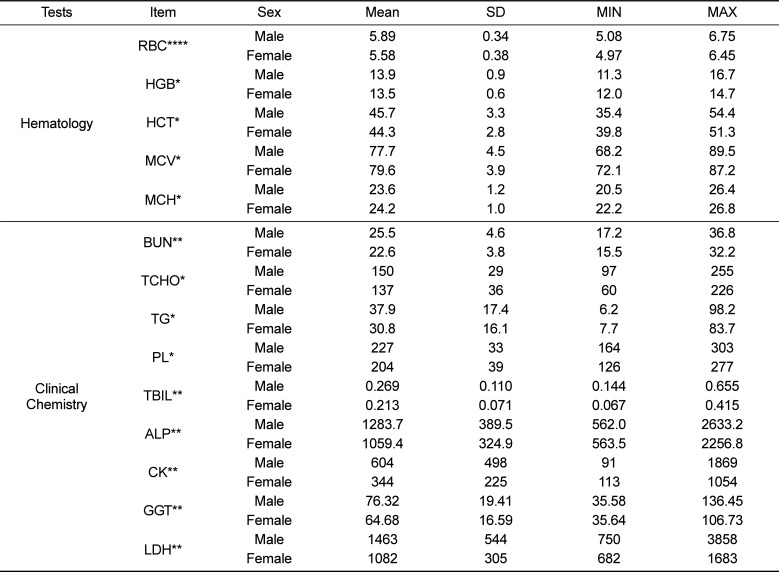
In the current study, sex-related differences were apparent in several hematological and biochemical parameters (Table 6). Red blood cell indices (RBC, HGB, and HCT) are important diagnostic parameters for anemia and internal hemorrhage. Consistent with previous data [1], our findings demonstrated that female monkeys have lower RBC, HGB, and HCT values than male monkeys. This tendency observed in red blood cell parameters was also observed in Sprague-Dawley rats and could be associated to the differential oxygen demand in relation to age and sex [16]. The clinical chemistry data showed that the most remarkable differences were observed in BUN, TBIL, ALP, CK, GGT, and LDH values (male vs. female, P<0.01). These indices showed sex-related difference attributed to the variation between male and female subjects in protein catabolism, food intake (BUN), mass of skeletal muscle (CK and LDH), specificity of tissue distribution (ALP), increased microsomal enzyme production, poor health condition (GGT) [3], and different rates of bilirubin metabolism (TBIL).
In conclusion, there are significant similarities between studies in several parameters values including most blood indices; however, some parameters (e.g., LDH, ALP, and CK) displayed large inter-study variability. This study provides valuable supplementation to the less extensively studied clinical chemistry (e.g., PL, LDL, HDL, CRP, and C3), coagulation (FIB), and urinalysis parameters. Our results will thus provide supplemental information on reference data of clinical pathological parameters in cynomolgus monkeys.
References
1. Xie L, Xu F, Liu S, Ji Y, Zhou Q, Wu Q, Gong W, Cheng K, Li J, Li L, Fang L, Zhou L, Xie P. Age- and sex-based hematological and biochemical parameters for Macaca fascicularis. PLoS One. 2013; 8(6):e64892. PMID: 23762263.
2. Drevon-Gaillot E, Perron-Lepage MF, Clément C, Burnett R. A review of background findings in cynomolgus monkeys (Macaca fascicularis) from three different geographical origins. Exp Toxicol Pathol. 2006; 58(2-3):77–88. PMID: 16984807.
3. Pritam S, Sahota JAP, Jerry F. Hardisty, Chirukandath Gopinath. Toxicologic Pathology: Nonclinical Safety Assessment. London: Taylor & Francis Group;2013. p. 154–159.
4. Koga T, Kanefuji K, Nakama K. Individual reference intervals of hematological and serum biochemical parameters in cynomolgus monkeys. Int J Toxicol. 2005; 24(5):377–385. PMID: 16257857.

5. Schuurman HJ, Smith HT. Reference values for clinical chemistry and clinical hematology parameters in cynomolgus monkeys. Xenotransplantation. 2005; 12(1):72–75. PMID: 15598276.

6. Zeng XC, Yang CM, Pan XY, Yao YS, Pan W, Zhou C, Jiang ZR, Chang Y, Ma J. Effects of fasting on hematologic and clinical chemical values in cynomolgus monkeys (Macaca fascicularis). J Med Primatol. 2011; 40(1):21–26. PMID: 20727063.
7. Perretta G, Violante A, Scarpulla M, Beciani M, Monaco V. Normal serum biochemical and hematological parameters in Macaca fascicularis. J Med Primatol. 1991; 20(7):345–351. PMID: 1787529.
8. Bonfanti U, Lamparelli D, Colombo P, Bernardi C. Hematology and serum chemistry parameters in juvenile cynomolgus monkeys (Macaca fascicularis) of Mauritius origin: comparison between purpose-bred and captured animals. J Med Primatol. 2009; 38(4):228–235. PMID: 19236562.
9. Lugo-Roman LA, Rico PJ, Sturdivant R, Burks R, Settle TL. Effects of serial anesthesia using ketamine or ketamine/medetomidine on hematology and serum biochemistry values in rhesus macaques (Macaca mulatta). J Med Primatol. 2010; 39(1):41–49. PMID: 19878432.
10. Kim CY, Lee HS, Han SC, Heo JD, Kwon MS, Ha CS, Han SS. Hematological and serum biochemical values in cynomolgus monkeys anesthetized with ketamine hydrochloride. J Med Primatol. 2005; 34(2):96–100. PMID: 15860116.

11. Yoshida T, Suzuki K, Cho F, Honjo S. [Age-related changes in hematological and serum biochemical values in cynomolgus monkeys (Macaca fascicularis) bred and reared using the indoor individually-caged system]. Jikken Dobutsu. 1986; 35(3):329–338. PMID: 3770085.
12. Syakalima M, Takiguchi M, Yasuda J, Hashimoto A. The age dependent levels of serum ALP isoenzymes and the diagnostic significance of corticosteroid-induced ALP during long-term glucocorticoid treatment. J Vet Med Sci. 1997; 59(10):905–909. PMID: 9362039.

13. Evans GO. Animal clinical chemistry. London: Taylor & Francis;2009. p. 257–258.
14. O'Brien PJ. Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology. 2008; 245(3):206–218. PMID: 18249481.
15. Chen Y, Qin S, Ding Y, Wei L, Zhang J, Li H, Bu H, Lu Y, Cheng J. Reference values of clinical chemistry and hematology parameters in rhesus monkeys (Macaca mulatta). Xenotransplantation. 2009; 16(6):496–501. PMID: 20042049.
16. Petterino C, Argentino-Storino A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol. 2006; 57(3):213–219. PMID: 16343876.

Table 1
Parameters and analytical methods
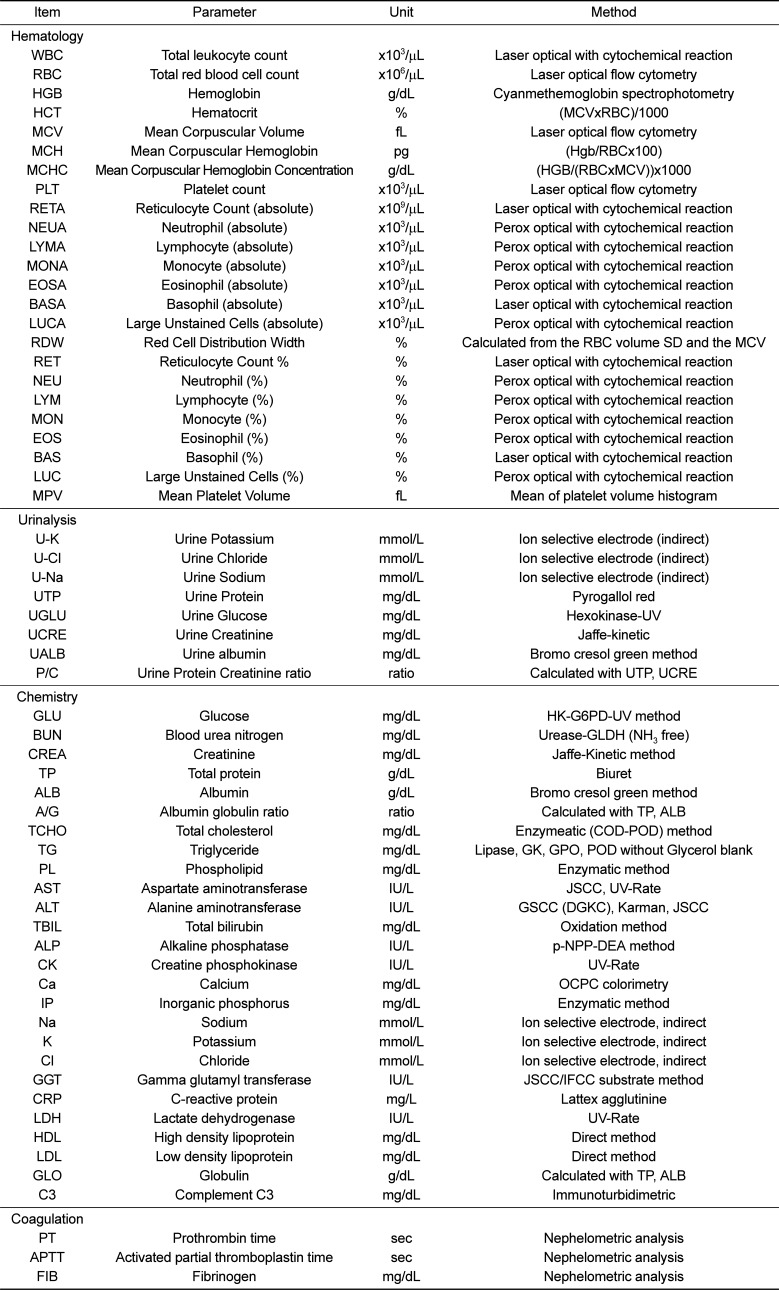
Table 2
Reference values of hematologic and urinalysis parameters for male cynomolgus monkeys
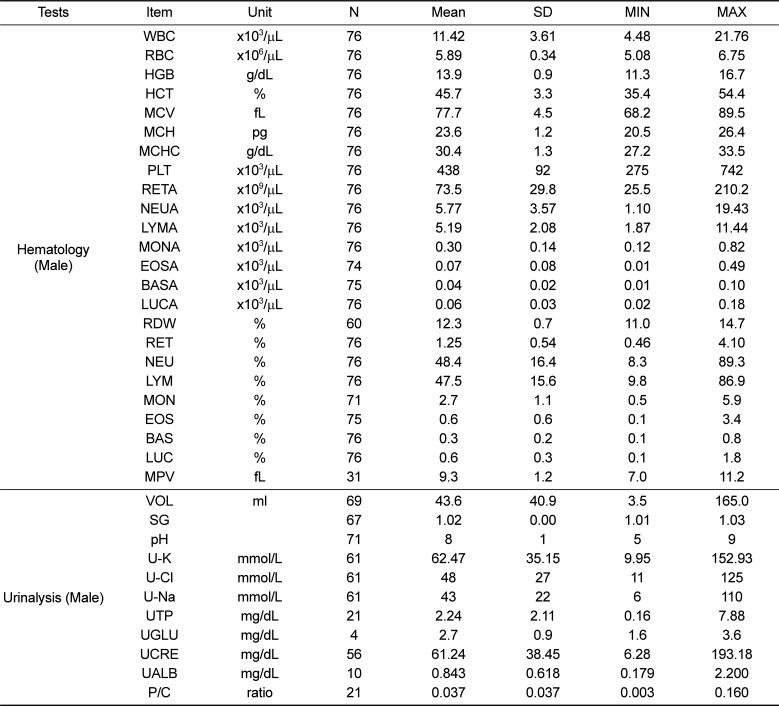
Table 3
Reference values of clinical chemistry and coagulation test parameters for male cynomolgus monkeys
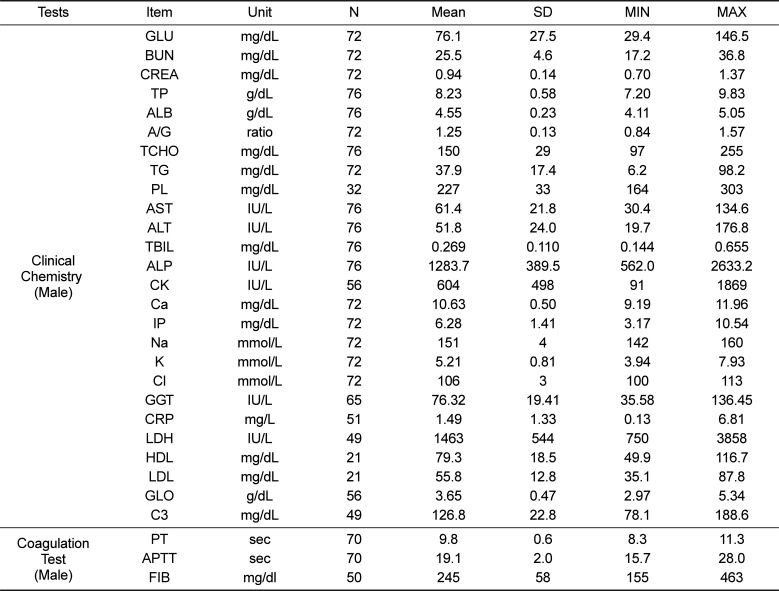
Table 4
Reference values of hematologic and urinalysis parameters for female cynomolgus monkeys
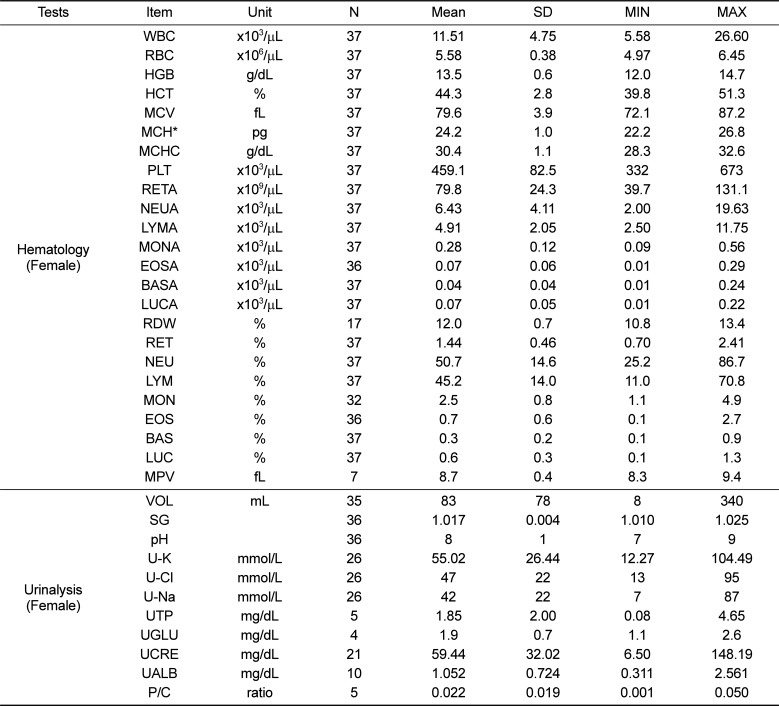
Table 5
Reference values of clinical chemistry and coagulation test parameters for female cynomolgus monkeys
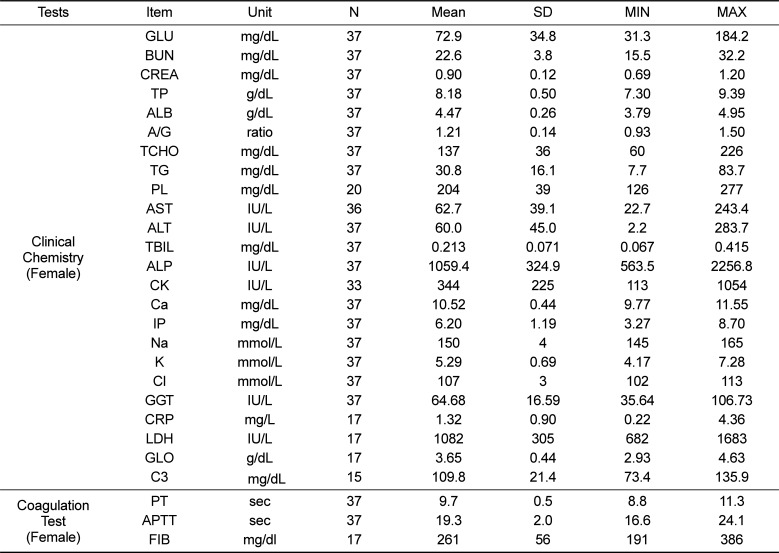
Table 6
Reference values of statistically significant sex differences




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download