This article has been
cited by other articles in ScienceCentral.
Abstract
A 19-month-old male German shepherd dog was presented with a hindlimb footpad mass. The mass was excised and histopathology was performed. Grossly, the cut section of the mass had multiple well-circumscribed nodules with a chalky appearance. Histopathologically, basophilic-stained calcium deposits of various sizes were distributed from dermis to subcutis, surrounded by epithelioid cells, multinucleated giant cells, and reactive fibroblasts. Myxoid metaplasia, calcium deposits in hair follicles, and psammoma-like bodies were also found. These histopathologic observations will greatly help to understand the pathogenesis of calcinosis circumscripta.
Go to :

Keywords: Calcinosis circumscripta, histopathologic diagnosis, myxoid change, Psammoma-like body
Calcinosis circumscripta is an uncommon disorder characterized by calcium deposits in soft tissue. It is also known as tumoral calcinosis, calcinosis cutis, calcium gout, or lipocalcinosis [
12]. It occurs most often in young large-breed dogs, particularly those 2 years of age, with German shepherd dogs being predisposed. The condition is characterized by the formation of nodules on sites of previous trauma, pressure points, and bony prominences, including the footpad, tongue, spine, salivary gland, and aorta. A retrospective pathological study of 77 cases in dogs revealed that the most common location of calcinosis circumscripta lesions is the hindlimb (50% of affected dogs) [
1]. Calcinosis is classified into 3 groups: dystrophic, metastatic, and idiopathic calcinosis [
13]. Dystrophic calcinosis occurs in a specific area of tissue damage, which may be due to injury, necrosis, inflammation, or neoplasia. Serum calcium and phosphate levels are normal in this type. Metastatic calcinosis is associated with abnormal calcium or phosphate metabolism, such as hypercalcemia or hyperphosphatemia derived from chronic renal failure, end-stage renal disease, or vitamin D toxicosis. Idiopathic calcinosis occurs without identifiable causative factors. In small animals, dystrophic and idiopathic calcinosis occurs more frequently than metastatic calcinosis [
1]. Herein, we describe the histopathologic features of calcinosis circumscripta in canine footpad, apropos of pathogenesis.
A 19-month-old male German shepherd dog presented with a hindlimb footpad mass. Grossly, a round, protruding mass was observed in the footpad of the hindlimb (
Figure 1A). The consistency was solid or firm. The excised mass was approximately sized 2×3 cm in diameter. The cut section contained chalky calcified nodules of various sizes (
Figure 1B).
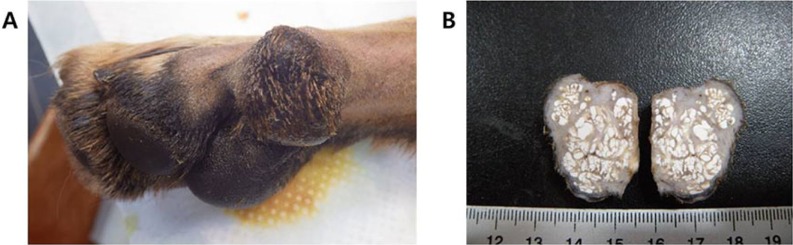 | Figure 1Gross findings. (A) A round mass protrudes from the footpad of the hindlimb. (B) Chalky calcified nodules of various sizes are observed in the cut section of the mass.
|
The mass was excised and sent to the Department of Veterinary Pathology, Kyungpook National University, for histological examination. The sample was fixed in 10% neutral buffered formalin, routinely processed, and embedded in a paraffin block. The paraffin block was trimmed and sectioned at a thickness of 4 µm. The sections were stained with hematoxylin-eosin (H&E).
Histopathologically, basophilic-stained calcium deposits of various sizes were extensively distributed from dermis to subcutis. The mineral deposits were separated by abundant fibrous connective tissue. The epidermis was moderately hyperplastic, with acanthosis and orthokeratotic hyperkeratosis (
Figure 2A). Calcification nodules were surrounded by epithelioid cells, multinucleated giant cells, and reactive fibroblasts (
Figure 2B,C). Calcium deposits were also observed in hair follicles, and the normal structure of hair follicles was lost (
Figure 3A,B). Psammoma-like bodies, a form of dystrophic calcification that appears as round, lamellated, concentric microscopic calcific collections, were found within fibrous connective tissue (
Figure 3C,D). Basophilic-stained myxoid metaplasia was observed in the surrounding matrix tissue (
Figure 3E,F).
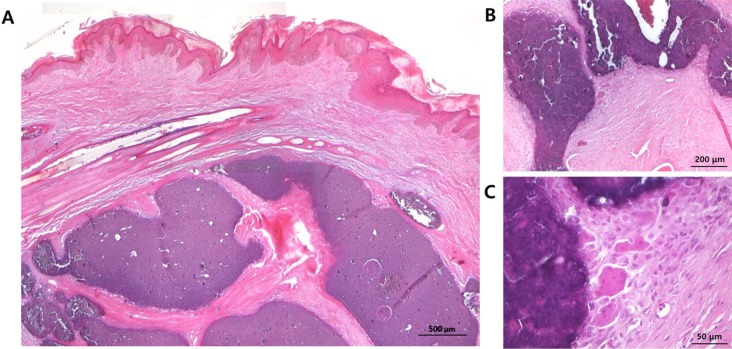 | Figure 2Microscopic findings of calcified nodules. (A) Basophilic-stained calcified nodules of various sizes are distributed from dermis to subcutis (original magnification 50×, scale bar=500 µm). (B, C) Calcification nodules are surrounded by epithelioid cells, multinucleated giant cells, and reactive fibroblasts (original magnifications 100× and 400×, scale bars= 200 and 50 µm).
|
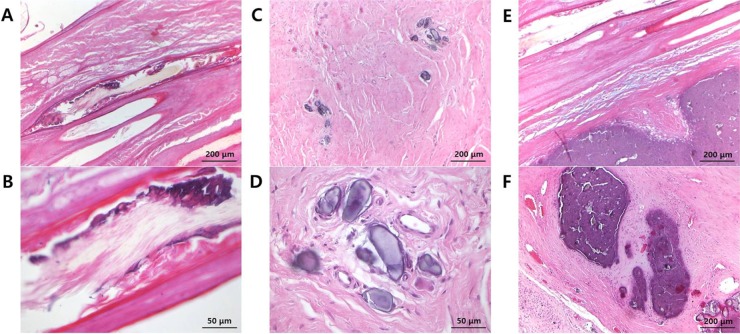 | Figure 3Microscopic findings of other calcium deposits. (A, B) Hair follicles lack normal structure and have calcium deposits (original magnifications 100× and 400×, scale bars=200 and 50 µm). (C, D) Psammoma-like bodies are found within fibrous connective tissue (original magnifications 100× and 400×, scale bars=200 and 50 µm). (E, F) Basophilic-stained myxoid metaplasia is observed in the surrounding matrix tissue (original magnification 100×, scale bar=200 µm).
|
According to a study of 77 dogs with calcinosis circumscripta, lesions could be microscopically divided into three categories according to age [
1]. Lesions of all stages had round-to-irregular shaped, variably sized deposits of amorphous to granular basophilic material. Stage I (early lesions) was characterized by minimal peripheral granulomatous reaction. Calcium deposits were found freely in normal tissue or surrounded by a thin layer of connective tissue with minimal or without inflammatory reaction. Stage II (intermediate lesions) had mild-to-moderate granulomatous reaction, epithelioid macrophages admixed with fewer small to large multinucleated giant cells, and occasional inflammatory cells, and was associated with variable amounts of fibroplasia. Stage III (late lesions) showed prominent granulomatous reaction, inflammatory mononuclear cells, and thick layers of fibrous connective tissue. In several cases, osseous and chondroid metaplasia was associated. According to this histopathological staging method, the lesions of the present case were categorized as stage II (intermediate lesions), characterized by moderate granulomatous reaction.
In this case, not only calcified nodules but also hair follicle calcification, psammoma-like bodies, and myxoid metaplasia were observed. Psammoma bodies, which are concentric lamellated calcified structures, are observed most commonly in papillary thyroid carcinoma, meningioma, and papillary serous cystadenocarcinoma of the ovary, but have been reported rarely in other neoplasms and nonneoplastic lesions [
4]. Psammoma bodies are said to indicate a process of dystrophic calcification, in which deposition occurs locally in nonviable or dying tissues with normal serum levels of calcium and in the absence of derangements in calcium metabolism [
4]. Dystrophic calcification has two major phases: initiation (nucleation) and propagation, which can occur intracellularly or extracellularly. In the initiation phase, dead and dying cells can no longer regulate the influx of calcium into the cytosol, and calcium accumulates in the mitochondria. In the propagation phase, calcium accumulations in single dead and dying cells act as seed crystals that become encrusted with more mineral deposits; progressive acquisition of outer layers may then create a lamellated configuration, giving rise to psammoma bodies [
4]. Myxoid change is another degenerative phenomenon found in a variety of diseases and tumors [
5678], although its mechanism remains speculative. Myxoid degeneration results from accumulation of mucin in intracellular or extracellular loci. It may occur in inflammatory conditions that cause epithelial hypersecretion after acute injury, or in conditions associated with late injury and repair, in which fibroblasts overproduce mucin. In addition, Scranton et al. demonstrated that injury is the most likely etiological factor of mucoid degeneration in the ligaments of athletes [
8]. The present case is calcinosis circumscripta occurring in the footpad of a German shepherd. The calcification process may be enhanced by the active calcium metabolism of a large, rapidly growing dog. In addition, the location suggested a dystrophic form of calcification, related to a pressure point. This was supported by histopathologic findings of psammoma bodies and myxoid change, both of which suggest a process of dystrophic calcification and degeneration.
The causes of calcinosis circumscripta include idiopathic (often with a genetic component), dystrophic (following tissue damage), or metastatic (associated with high serum calcium or phosphate) calcification. Other causes include prolonged glucocorticoid therapy, hyperadrenocorticism, chronic renal disease, fungal infection, hyperparathyroidism, and vitamin D toxicosis. Treatment of calcinosis circumscripta consists of surgical removal of the calcified tissue. If the primary causes are not resolved, it requires constant attention even after surgical resection.
In conclusion, we describe a case of calcinosis circumscripta in the footpad of a German shepherd. Histopathologic findings include not only calcified nodules but also calcified hair follicles, myxoid change, and psammoma-like bodies. These findings will increase understanding of the pathogenesis of dystrophic calcinosis circumscripta, and facilitate its differential diagnosis from metastatic and idiopathic types.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download