Abstract
Anticoccidial effects of the Plantago asiatica extract (PAE) were evaluated in chickens following oral infection with Eimeria (E.) tenella. This study was conducted on the 3-day-old chickens (n=30). Those animals were divided with 3 groups; PAE 0.1% treated/infected (n=10), PAE untreated/infected (n=10) and non-infected control (n=10). Chickens were fed a standard diet supplemented with or without PAE for 1 week prior to infection with E. tenella (10,000 sporulated oocysts per chicken). The effects of PAE on E. tenella infection were assessed by two parameters; fecal oocysts shedding and body weights gain. The PAE-fed chickens produced significantly reduced fecal oocysts (P<0.05) when compared to the E. tenella-infected group fed standard diet. Also, PAE-based diet, improved body weight loss caused by E. tenella infection. Our data demonstrated that PAE had remarkable anticoccidial activities against E. tenella. This finding might have implications for the development of anticoccidial drug. This study is the first to demonstrate anticoccidial effect of PAE on Eimeria parasites.
Coccidiosis is induced by Eimeria species infection and an important parasitic disease of poultry [4]. Losses include mortality, morbidity and cost of preventative or therapeutic drugs and/or vaccination. In addition, many of the in-feed medications commonly used for prevention of infections with Eimeria species have become less effective because some strains of parasites have developed reduced susceptibility to anticoccidials [23]. This suggests that coccidiosis is likely to have a greater impact on the profitability of broiler meat production in the future [23].
Many biological activities in different species of the family Plantaginaceae have been reported. Traditionally, Plantago asiatica L. has long been used for the treatment of many diseases including wounds, bronchitis, cholesterolemia, chronic constipation and diarrhea [101621]. This herbal medicine has also been shown to possess antileukemia, anticarcinoma and antiviral activities, as well as activities which modulate cell-mediated immunity [3]. The seeds of Plantago asiatica L. were regarded as orthodox product in ancient Chinese medical literature. Its chemical components, including phenylethanoid glycosides [12], phenolics [17] and various polysaccharides [20] have been widely studied. It was found that the seeds of Plantago asiatica L. could enhance the immune function of the immunosuppressant mice [24].
Although a variety types of natural products have been investigated in search for alternative controls of coccidiosis in chickens [4], the effects of Plantago asiatica on Eimeria infection has not been studied.
The present study is aimed to investigate the anticoccidial effects of Plantago asiatica extract (PAE) in chickens following oral infection with Eimeria (E.) tenella.
The dry mass of the Plantago asiatica was purchased from an Oriental Pharmacy (Iksan, Korea), was according to the standard as mentioned in Korean Pharmacopoeia and Korean Herbal Pharmacopoeia, which are the official compendia of standard. The procedure for preparing PAE was as follows. The air-dried mass of Plantago asiatica (100 g) was cut into pieces and extracted twice with 50% (v/v) ethanol (Six times as much as the weight of the dried plants) for 3 hours at 80℃. After filtration through a 400-mesh filter cloth, the filtrate was refiltered through filter paper (Whatman, No. 5) and concentrated on a rotary evaporator (EYELA, Tokyo, Japan) and the concentrated filtrate was evaporated to dryness under vacuum with freezing dryer (Labconco, USAFinally, the solid residue was collected, placed in sealed bottles and stored at −20℃. The extract yield of Plantago asiatica with 50% ethanol was 15.55%.
Plantamajoside, which was used for the standard material of PAE composition, was purchased from the Natural Product Bank, Institute for Korea Traditional Medical Industry (Geong-San, Korea). Plantago asiatica is a member of the Plantaginaceae family, is widely distributed in East Asia, used in Chinese medicine, where its leaf is called Plantaginis Herba and its seed is Plantaginis Semen [2], and has been commercialized as an extract and as a dietary supplement in various forms. A previous report discussed the isolations of plantamajoside, plantagin and aucubin from P. asiatica, as well as the various biological properties of these compounds, including antibacterial, antiallergic, antiinammatory, antioxidative and lipoxygenase inhibitory activity [15].
Figure 1 shows the chemical structures of phenylethanoid glycosides (PhGs) including acteoside and plantamajoside. The plantamajoside composition of PAE was analyzed by LC. Waters ACQUITY LC system (Waters Corp., Milford, USA) was used for LC system. The column was C18 type ACQUITY UPLC BEH (2.1×50 mm, 1.7 µm, Waters Corp., Milford, USA). A Waters Nova Pack C-18 column (ACQUITY UPLC BEH (2.1×50 mm, 1.7 µm, Waters Corp., Milford, USA) was employed. The wavelength of the UV detector was set at 300 nm. The column temperature was set at 30℃ with a flow rate of mobile phase at 0.6 mL/min (0.1% H3PO4/Acetonnitrile).
This study was conducted on the 3-day-old chickens (n=30) in the animal facility of Center for Animal Resources Development, Wonkwang University, Korea. Animals were acclimatized and kept in an animal facility room with regulated temperature (28±2℃), humidity (50±5%) and light/dark cycle (12/12 hours). The animals were fed commercial post-broiler diet without antibiotics and coccidiostat (Hanil Feed Co., Yongin, Korea) and tab water ad libitum. The chickens were kept in wire-floored grower cages during study period. All studies were performed in accordance with the Guide for Animal Experimentation by Wonkwang University and approved by the Institutional Animal Care and Use Committee of Wonkwang University. All efforts were made to minimize pain or discomfort of animals used.
Anticoccidial effects of PAE were evaluated in chickens following oral infection with E. tenella. This study was conducted on the 3-day-old chickens (n=30). Those animals were divided with 3 groups; PAE 0.1% treated/infected (n=10), PAE untreated/infected (n=10) and non-infected control (n=10). We decided the dose of PAE following as the recommended feed additive concentration. Chickens were fed a standard diet supplemented with or without PAE for 1 week prior to infection with E. tenella (10,000 sporulated oocysts per chicken). The effects of PAE on E. tenella infection were assessed by two parameters, fecal oocysts shedding and body weights gain.
Oocysts of E. tenella were cleaned by flotation on 5.25% sodium hypochlorite and washed three times with phosphate buffered saline. E. tenella was provided kindly by Professor Wongi Min at Gyeongsang National University in Korea. Chickens were treated orally by gavages using a 24 gauge, mouse stainless steel animal feeding tube (Popper & Sons, Inc., New York, USA) attached to a 3 mL syringe. The oral infectious dose of has been approximated 104 oocysts of E. tenella in 1 mL of saline. The control chickens (n=10) received saline through the same route.
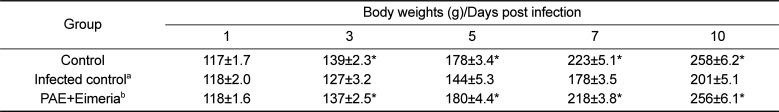
During the study period, the animals were checked twice daily for morbidity and mortality. Further, we compared clinical signs and body weight changes of experimental animals. Body weights were individually measured for 2 weeks before infection and for 10 days post-infection.
Fecal materials were collected from 6 to 10 days post-infection. The fecal samples were analyzed for the presence of coccidial oocysts using a standard fecal flotation technique [8]. Briefly, 5 mL from each sample was pelleted by centrifugation at 1500×g for 5 min. The resulting pellet was resuspended in saturated sodium chloride (aqueous), passed through a 1 mm mesh size sieve to remove coarse fecal debris. The resulting filtrate was used in a standard gravity vial fecal flotation using 22 mm×22 mm coverslips. After flotation, the coverslip was mounted on a slide and examined in its entirety for the presence of coccidial oocysts. Total number of oocysts was calculated using the following formula: [total number of oocysts=oocyst count×dilution factor×(fecal sample volume/counting chamber volume)/number of birds per cage].
Differences in mean oocysts production and mean weight gain between the 4 groups were tested by using one-way analysis of variance (ANOVA; GraphPad InStat; GraphPad Software Inc., San Diego, CA) and considered significant at P<0.05.
The extract yield of Plantago asiatica with 50% ethanol was 15.55%. We analyzed PAE composition by LC. The concentration of plantamajoside in PAE was 479.8 µg/mL.
The effects of PAE on E. tenella infection were assessed by two parameters, fecal oocysts shedding and body weight gain. PAE-fed chickens produced significantly reduced fecal oocysts (P<0.05) when compared to the E. tenella-infected group fed standard diet. Also, PAE-based diet, improved body weight loss caused by E. tenella infection.
The results showed that, compared to untreated controls, chickens treated with PAE had significantly decreased fecal oocysts shedding and showed strong anticoccidial activities (P<0.01).
As shown in Table 1, oocyst shedding was significantly higher in the inoculated chickens than in the control chickens (P<0.05). The number of fecal oocysts shed was highest on day 7 post-inoculation (Table 1). Moreover, body weight gain was lesser in the animals in the inoculated group than in the animals in the control group (Table 2).
Coccidiosis of domestic fowl is a worldwide disease caused by obligatory intracellular protozoa of the genus Eimeria. It is responsible for important economic losses in poultry production. E. tenella is important pathogen causing avian coccidiosis in laboratory avian animals and known to affect influencing experimental results obtained with contaminated animals [411]. The disease is characterized by enteric lesions of variable extent and severity, reducing the absorptive function of the intestinal mucosa, thus leading to weight loss, diarrhea, poorer feed conversion and a higher mortality in the affected flocks [22].
The results of this study showed that PAE had a strong anticoccidial effect on E. tenella. PAE contains a large amount of phenylethanoid glycosides. The major bioactive constitute of P. asiatica are phenylethanoid glycosides (PhGs) including acteoside and plantamajoside as shown in Scheme 1. PhGs from many plants have been extensively studied for their various biological functions such as anti-hepatotoxic [26], anti-inflammatory, antinociceptive activities [20], improving sexual function [2528] and antioxidant activity [5927]. The plantaginin, plantamajoside, acetoside and 6-hydroxyluteolin 7-glucoside isolated from P. asiatica were the chief compounds [15147] and they had various biological activities [63]. The plantamajoside, in particular, was reported to show antibacterial activity against Staphylococcus aureus [18], inhibition activity against cAMP phosphodiesterase and 5-lipoxygenase [17], and antioxidant activity on ADP+ NADPH-induced lipid peroxidation in rat liver microsome [12]. Also, the plantamajoside is used as a marker compound in chemotaxonomic studies, as are other members of the dihydroxyphenylethyl glycoside family [131]. Theses phenylethanoid glycosides-included bioactive structures could be suggested a plausible explanation of the anticoccidial properties in this study.
In this study, anticoccidial effects of PAE were evaluated in chickens following oral infection with E. tenella. The PAE-fed chickens produced significantly reduced fecal oocysts (P<0.05) when compared to the E. tenella-infected group fed standard diet. Also, PAE-based diet, improved body weights loss caused by E. tenella infection. Our data demonstrated that PAE had remarkable anticoccidial activities against E. tenella. This finding might have implications for the development of anticoccidial drug. This study is the first to demonstrate anticoccidial effect of PAE on Eimeria parasites.
References
1. Andary PC, Motte-Florac ME, Gargadennec A, Wylde R, Heintz A. Les esters cafeiques du genre Plantago. Identification et valeur chimiotaxinomique. Plantes Med Phytother. 1988; 22:17–22.
2. Bae JH, Kim JE. Inhibitory effect of Plantago asiatica extracts on the growth of gastric and colon cell lines. Food Sci Biotechnol. 2004; 8:104–116.
3. Chiang LC, Chiang W, Chang MY, Lin CC. In vitro cytotoxic, antiviral and immunomodulatory effects of Plantago major and Plantago asiatica. Am J Chin Med. 2003; 31:225–234. PMID: 12856861.
4. Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 2006; 5(1):143–163. PMID: 16451116.

5. He ZD, Lau KM, Xu HX, Li PC, Pui-Hay But P. Antioxidant activity of phenylethanoid glycosides from Brandisia hancei. J Ethnopharmacol. 2000; 71(3):483–486. PMID: 10940587.

6. Ko SG, Koh SH, Jun CY, Nam CG, Bae HS, Shin MK. Induction of apoptosis by Saussurea lappa and Pharbitis nil on AGS gastric cancer cells. Biol Pharm Bull. 2004; 27(10):1604–1610. PMID: 15467204.

7. Komoda Y, Chujo H, Ishihara S, Uchida M. HPLC quantitative analysis of plantaginin in Shazenso (Plantago asiatica L.) extracts and isolation of plantamajoside. Tokyo Ika Shika Daigaku Iyo Kizai Kenkyusho Hokoku. 1989; 23:81–85. PMID: 2488968.
8. Lee HA, Hong S, Chung Y, Kim O. Sensitive and specific identification by polymerase chain reaction of Eimeria tenella and Eimeria maxima, important protozoan pathogens in laboratory avian facilities. Lab Anim Res. 2011; 27(3):255–258. PMID: 21998616.
9. Li J, Wang PF, Zheng R, Liu ZM, Jia Z. Protection of phenylpropanoid glycosides from Pedicularis against oxidative hemolysis in vitro. Planta Med. 1993; 59(4):315–317. PMID: 8372146.
10. Marlett JA, Fischer MH. The active fraction of psyllium seed husk. Proc Nutr Soc. 2003; 62(1):207–209. PMID: 12749348.

11. McDougald LR. Protozoal infections. In : Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editors. Diseases of Poultry. Ames, USA: Iowa State Press;2003. p. 973–991.
12. Miyase T, Ishino M, Akahori C, Ueno A, Ohkawa Y, Tanizawa H. Phenylethanoid glycosides from Plantago asiatica. Phytochemistry. 1991; 30:2015–2018.
13. Mølgaard P. Population genetics and geographical distribution of caffeic acid esters in leaves of Plantago major in Denmark. J Ecol. 1986; 74:1127–1137.
14. Murai M, Tamayama Y, Nishibe S. Phenylethanoids in the herb of Plantago lanceolata and inhibitory effect on arachidonic acid-induced mouse ear edema. Planta Med. 1995; 61(5):479–480. PMID: 7480214.
15. Nishibe S. The plant origins of herbal medicines and their quality evaluation. Yakugaku Zasshi. 2002; 122(6):363–379. PMID: 12087774.

16. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005; 100(4):936–971. PMID: 15784043.

17. Ravn H, Nishibe S, Sasahara M. Phenolic compounds from Plantago asiatica. Phytochemistry. 1990; 29:3627–3631.
18. Ravn H, Brimer L. Structure and antibacterial activity of plantamajoside, a caffeic acid sugar ester from Plantago major subsp. major. Phytochemistry. 1988; 27:2433–3437.
19. Samuelsen AB, Lund I, Djahromi JM, Paulsen BS, Wold JK, Knutsen SH. Structural features and anti-complementary activity of some heteroxylan polysaccharide fractions from the seeds of Plantago major L. Carbohydr Polym. 1999; 38:133–143.
20. Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 2002; 36(2):177–187. PMID: 11999386.

21. Singh B. Psyllium as therapeutic and drug delivery agent. Int J Pharm. 2007; 334:1–14. PMID: 17329047.

22. Stotish RL, Wang CC, Meyenhofer M. Structure and composition of the oocyst wall of Eimeria tenella. J Parasitol. 1978; 64(6):1074–1081. PMID: 739302.

23. Williams RB. Relative virulences of a drug-resistant and a drug-sensitive strain of Eimeria acervulina, a coccidium of chickens. Vet Parasitol. 2006; 135(1):15–23. PMID: 16361061.

24. Xie X, Fu Z, Xie M, Wan Y, Chen L, Wu J, Dai D. Experimental research of polysaccharide in the seeds of Plantago asiatica L. on immunological function in mice. The 3rd Traditional Chinese Medicine Immune Academic Seminar Hunan, China. 2006. p. 18–22.
25. Xie JH, Wu CF. Effect of ethanolic extract of Cistanche deserticola on the contents of monoamine neurotransmitters in rat brain. Chin Trad Herb Drug. 1993; 24:417–419.
26. Xiong Q, Hase K, Tezuka Y, Tani T, Namba T, Kadota S. Hepatoprotective activity of phenylethanoids from Cistanche deserticola. Planta Med. 1998; 64(2):120–125. PMID: 9525102.
27. Xiong Q, Kadota S, Tani T, Namba T. Antioxidative effects of phenylethanoids from Cistanche deserticola. Biol Pharm Bull. 1996; 19(12):1580–1585. PMID: 8996643.

28. Zong G, He W, Wu G, Chen M, Shen X, Shi M. Comparison between Cistanche deserticola Y. C. Ma and C, tubulosa (Shenk) Wight on some pharmacological actions. Zhongguo Zhong Yao Za Zhi. 1996; 21(7):436–437. PMID: 9642400.
Figure 1
Structures of PhGs extracted from P. asiatica. (1) Plantamajosides, R1=Rham; (2) acteoside, R1=Glc.
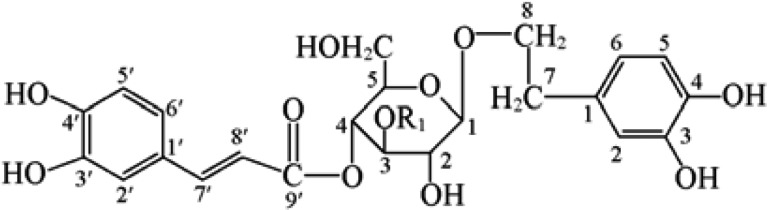
Table 1
Results of shedding oocysts number count in the feces of studied chickens
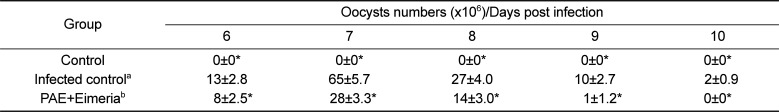
| Group | Oocysts numbers (×106)/Days post infection | ||||
|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | |
| Control | 0±0* | 0±0* | 0±0* | 0±0* | 0±0* |
| Infected controla | 13±2.8 | 65±5.7 | 27±4.0 | 10±2.7 | 2±0.9 |
| PAE+Eimeriab | 8±2.5* | 28±3.3* | 14±3.0* | 1±1.2* | 0±0* |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download