Abstract
Animal models for gastric ulcers produced by physical, pharmacological and surgical methods have been widely employed to evaluate therapeutic drugs and investigate the mechanism of action of this disease. ICR mice were selected to produce this model, even though several mice and rats have been widely used in studies of gastric ulcers. To compare the responses of ICR mice obtained from three different sources to gastric ulcer inducers, alterations in gastric injury, histopathological structure, and inflammation were measured in Korl:ICR (Korea NIFDS source), A:ICR (USA source) and B:ICR (Japan source) treated with three concentrations of ethanol (EtOH) (50, 70, and 90%) in 150 mM hydrochloric acid (HCl) solution. Firstly, the stomach lesion index gradually increased as the EtOH concentration increased in three ICR groups. Moreover, a significant increase in the level of mucosal injury, edema and the number of inflammatory cells was similarly detected in the EtOH/HCl treated group compared with the vehicle treated group in three ICR groups. Furthermore, the number of infiltrated mast cells and IL-1β expression were very similar in the ICR group derived from three different sources, although some differences in IL-1β expression were detected. Especially, the level of IL-1β mRNA in 50 and 90EtOH/HCl treated group was higher in Korl:ICR and A:ICR than B:ICR. Overall, the results of this study suggest that Korl:ICR, A:ICR and B:ICR derived from different sources have an overall similar response to gastric ulcer induced by EtOH/HCl administration, although there were some differences in the magnitude of their responses.
The gastric ulcer is a pathological lesion characterized by infiltration of neutrophils, increased necrosis, decreased blood flow, stimulation of oxidative stress and secretion of inflammatory mediators [1]. This disease is caused by various aggressive and defensive factors, including ethanol, gastric acid, acid-pepsin, reactive oxygen species, nonsteroidal anti-inflammatory drugs and Helicobacter pylori [23]. To date, the anti-ulcerogenic effects of various natural products such as Zuojin Pill [3], large yellow croaker swim bladder [4], Korea Red Ginseng [5], Coptidis Rhizoma [6], and Sasammaickmoondong-tang [7] have widely been investigated using several animal models for gastric ulcer.
Animal models for human diseases have also been applied to evaluate the preventive or curative effects of herbal medicines or chemical drugs, as well as explore the mechanism of action of candidate drugs with antiulcer activity, with each model showing some advantages and disadvantages [8]. These models can be induced by physiological, pharmacological or surgical methods. Many pharmacological compounds including ethanol (EtOH), gastric acid, histamine, reserpine, serotonin, diethyldithiocarbonate, methylene-blue, cysteamine, aspirin, ibuprofen, indomethancin and ferrous iron have been used to produce animal models for gastric ulcer, while water-immersion stress, pylorus-ligation and ischemiareperfusion can be considered a physiological and surgical procedure for developing gastric ulcer models [8]. Among these, the EtOH-induced model is most widely used to investigate the efficacy of potential drugs because it is independent of gastric acid secretion and resembles acute gastric ulcers in humans [9]. Furthermore, several strains of rodents including SD rats, Wistar rats, C57BL/6 mice, ICR mice and BALB/c mice have been utilized to conduct most experiments in studies for gastric ulcer, although ICR mice are most commonly used for these studies [25101112]. However, most used only one strain of one animal species to produce animal models for gastric ulcer and evaluate the anti-gastric ulcer activity of some compounds and extracts. No studies have provided scientific evidence for comparative analysis of the response of specific mice strain derived from different sources to chemicals that cause gastric injury.
Therefore, the present study was conducted to compare the response of ICR mice (Korl:ICR, A:ICR and B:ICR) derived from three difference sources to EtOH/hydrochloric acid (HCl) induced gastric ulcer. Our results provide the first scientific evidence of a very similar response to EtOH/HCl induced gastric ulcer in the stomach of Korl:ICR, A:ICR and B:ICR, although there were slight differences in the magnitude of these effects.
The protocols for the animal experiment were carefully reviewed for ethical and scientific care procedures and approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2015-0916). Six-week-old male ICR mice were obtained from three difference sources. Korl:ICR mice were kindly provided by the Department of Laboratory Animal Resources in the National Institute of Food and Drug Safety Evaluation (NIFDS, Cheongju, Korea). The other two ICR groups of ICR mice were purchased from different vendors located in the United States (A:ICR) and Japan (B:ICR). All mice were provided with ad libitum access to standard irradiated chow diet (Samtako Inc., Osan, Korea) consisting of moisture (12.5%), crude protein (25.43%), crude fat (6.06%), crude fiber (3.9%), crude ash (5.31%), calcium (1.14%), and phosphorus (0.99%) and water. During the experiment, mice were maintained in a specific pathogen-free state under a strict light cycle (lights on at 08:00 hours and off at 20:00 hrs) at 23±2℃ and 50±10% relative humidity. The mice were housed in the Pusan National University-Laboratory Animal Resources Center accredited by the Korea Ministry of Food and Drug Safety (MFDS) in accordance with the Laboratory Animal Act (Accredited Unit Number-000231) and AAALAC International according to the National Institutes of Health guidelines (Accredited Unit Number; 001525).
Moreover, alterations in body weight were measured using an electronic balance (Mettler Toledo, Greifensee, Switzerland) once a week according to the MFDS guideline.
The gastric lesions were induced by traditional methods, with some modifications as described in previous studies [1314]. After 24 h of food starvation, mice (six-week-old, n=28) from Korl:ICR, A:ICR and B:ICR group were randomly divided into one of four groups. The first group (vehicle group, n=7) received a constant volume of dH2O, while other groups were administered 0.2 mL of three different concentrations EtOH in 150 mM HCl solution; 50% EtOH (50EtOH/HCl treated group), 70% EtOH (70EtOH/HCl treated group) and 90% EtOH (90EtOH/HCl treated group). After 1 h, all of the animals in each group were euthanized using a chamber filled with CO2 and gas and stomach samples were collected.
To analyze the macroscopic gastric lesions, the stomachs were excised from ICR mice of each group and opened along the greater curvature as previously described [21314]. After repeatedly rinsing with 1× PBS, the inside of the stomach was photographed using a Canon® digital camera (Canon Inc., Japan). The area of visible erosive lesions was measured using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany) and the images were analyzed using the Image J software (National Institutes of Health, Maryland, USA). The lesion index was calculated as previously described [15]; Lesions index (%)=(gastric injury area/gastric total area of mice) ×100%.
Stomach tissues were excised from ICR mice, fixed in 10% formalin, embedded in paraffin wax, routinely processed, and then sectioned into 4 µm thick slices. Next, the stomach sections were stained with hematoxylin and eosin (H&E), after which they were examined by light microscopy for alterations in histological structure. Furthermore, their lesions were analyzed according to previously defined criteria [616]. Briefly, a 1 cm segment of each histological section was assessed for the mucosal injury including epithelial cell loss (score: 0-3), edema (score: 0-3), and the presence of inflammatory cells (score: 0-3).
In addition, the infiltration of mast cells into stomach tissue was detected by staining with toluidine blue as previously described [17]. After deparaffinization and dehydration, stomach sections were stained with 0.25% toluidine blue (Sigma-Aldrich Co., Missouri, USA) and examined by light microscopy for the presence of mast cells. The number of cells per specific area was determined using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany).
For RT-PCR analysis, stomachs were frozen in liquid nitrogen, then chopped with scissors and homogenized in RNAzol B solution (Tet-Test Inc., Texas, USA). The isolated RNA was subsequently measured using a Biospecnano spectrophotometer (Dong-il Shimadzu, Korea). Expression of the transgenes was assessed by RT-PCR using 5 µg of the total RNA from each tissue. Next, 500 ng of oligo-dT primer (Invitrogen, California, USA) was annealed at 70℃ for 10 min. Complementary DNA, which was used as the template for further amplification, was synthesized by the addition of dATP, dCTP, dGTP, and dTTP with 200 units of reverse transcriptase. Subsequently, 10 pmol of the sense and antisense primers were added, and the reaction mixture was subjected to 32 cycles of amplification in a Perkin-Elmer Thermal Cycler as follows: 1 min at 94℃, 1 min at 62℃, and 1 min at 72℃. In each case, negative-RT controls were included to differentiate between DNA and RNA products. RT-PCR was also conducted using β-actin specific primers to ensure RNA integrity. The primer sequences for IL-Iβ sense and antisense primers were as follows: 5'-GCA CAT CAA CAA GAG CTT CAG-3' and 5'-GGT ACA TCA GCA CCT CAC AAG CAG AG-3'. The sequences of the β-actin sense and antisense primers were 5'-TGG AAT CCT GTG GCA TCC ATG AAA C-3' and 5'-TAA AAC GCA GCT CAG TAA CAG TCC G-3', respectively. All samples were analyzed in triplicate, and the final PCR products were separated by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
One-way ANOVA was used to identify significant differences between vehicle and EtOH/HCl treated groups (SPSS for Windows, Release 10.10, Standard Version, Chicago, Illinois, USA). All values are reported as the means±standard deviation (SD) and a P value of <0.05 was considered significant.
The body weights of ICR mice were measured before and after EtOH/HCl administration to determine the animal toxicity. There were no significant differences in body weights of mice between the vehicle treated group and EtOH/HCl treated group, although the body weights of A:ICR mice were slightly lower than those of the other two groups. The body weight was also maintained at a constant level in all EtOH/HCl treated groups, regardless of the concentration of EtOH (Table 1). Furthermore, these consistencies in body weight were observed in all ICR groups. Overall, these results show that EtOH/HCl administration to induce gastric ulcer did not affect cause significant alterations in body weight of any ICR group.
To compare the response of gastric lesions of ICR mice provided from three different sources to EtOH/HCl administration, alterations in the stomach morphology and gastric lesions were measured in the stomach of Korl:ICR, A:ICR and B:ICR mice treated with three different concentrations of EtOHs/HCl. Hemorrhagic lesions inside the stomach appeared after administration of 50EtOH/HCl, and more severe lesions were noted in response to 70EtOH/HCl and 90EtOH/HCl. The lesion index was markedly increased in the EtOH/HCl treated group compared with the vehicle treated group. These alterations were dependent on the increase in EtOH concentration. Similar enhancements of the lesion index in the stomach were observed in Korl:ICR, A:ICR and B:ICR mice, with the highest lesion index being observed in the 90EtOH/HCl treated group (Figure 1). Therefore, the present results indicate that Korl:ICR, A:ICR and B:ICR showed similar patterns of gastric ulcer formation to EtOH/HCl administration.
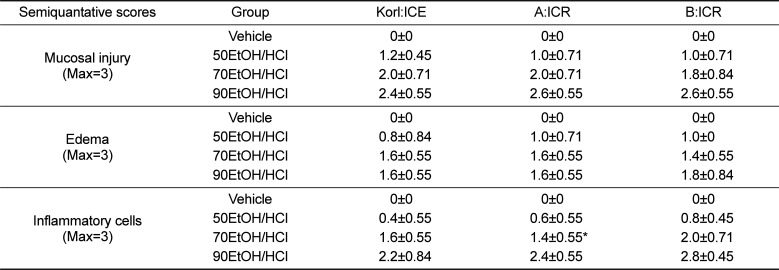
To compare alterations in the stomach histology of ICR mice from three different sources in response to EtOH/HCl administration, alterations in the histological structure of the stomach were observed in Korl:ICR, A:ICR and B:ICR administered with EtOH/HCl. A significant increase in loss of the mucous membrane, necrosis and congestion in the stomach tissue was observed in the EtOH/HCl treated group relative to the vehicle treated group. No significant differences in histopathological structure were observed among the three ICR groups (Figure 2). The levels of mucosal injury, edema and number of inflammatory cells were higher in the 50EtOH/HCl treated group than the vehicle treated group. These levels were further increased in the 70EtOH/HCl and 90EtOH/HCl treated group, although the edema level remained constant in the 70EH/HCl and 90EH/HCl treated group. Moreover, the ICR groups from three different sources showed similar patterns upon histopathological analysis (Table 2). Therefore, the above results suggested that the histopathological structure for gastric ulcer may be successfully induced by EtOH/HCl treatment in ICR mice from three different sources without significant differences.
To compare the inflammatory response in the stomach of ICR mice derived from three different sources to EtOH/HCl administration, alterations in the number of mast cells and IL-1β expression were measured in the stomach tissues of Korl:ICR, A:ICR and B:ICR treated with EtOH/HCl. A few mast cells in the region of gastric pits were stained blue in the vehicle treated group. However, the number of these cells was significantly higher in the EtOH/HCl treated group relative to the vehicle treated group, while the highest number was detected in the 90EtOH/HCl treated group. These alterations in mast cell infiltration were commonly observed in Korl:ICR, A:ICR and B:ICR without any significant difference (Figure 3). A different pattern was also observed in the expression of IL-1β, although their level was consistently maintained in some group. In 50EtOH/HCl or 90EtOH/HCl treated group, the expression of IL-1β transcripts was higher in Korl:ICR and A:ICR than B:ICR, while a constant level was maintained between Korl:ICR and A:ICR. However, 70EtOH/HCl treated group showed a similar level of IL-1β transcripts in all ICR group (Figure 4). Overall, the above results indicate that stomach inflammation may be successfully induced by EtOH/HCl administration in ICR mice from three different sources, although the magnitude of their response varied in some group.
Various animal models have been used for a long time in the drug discovery and development process to investigate pathophysiological characters of disease, to evaluate the action mechanism of drugs, to identify novel targets and biomarkers of drugs [18], to establish pharmacodynamics/pharmacokinetic relationships [1920], and to determine safety margins and toxicity [21]. Generally, these models can be produced by several methods, including direct injection of chemicals and biological agents into specific animals, as well as injection of recombinant DNA into fertilized eggs [22]. Many experimental models for gastric ulcer have been induced by combined injection of drug-induced vagal stimulation with necrotizing agents or anti-inflammatory drugs, as well by administration of Helicobacter pilori [23]. In this study, we used different concentrations (50, 70, and 90%) of EtOH in 150 mM HCl to produce an animal model for gastric ulcer and the differences in the response to these chemicals among ICR mice derived from three different sources were investigated. The results presented here provide the first evidence that Korl:ICR, A:ICR and B:ICR show a similar response to EtOH/HCl induced gastric ulcer, although some differences were detected in IL-1β expression.
Rodents including mice and rats have generally been selected over guinea pigs, rabbits, dogs, cats and primates to produce various models for human disease [24]. Among these animals, ICR mice were first established by Hauschka at the Institute for Cancer Research after the introduction of Swiss mice into the United States from Lausanne in Switzerland [25]. Since then, they have become the most commonly used research mice as an outbred stock. ICR mice are also widely used in toxicology (safety and efficacy testing), cancer research, aging, transgenesis experiments and gene mapping strategies [262728]. Meanwhile, ICR mice have commonly been applied to produce animal models for gastric ulcer, although comparative studies between animals derived from different sources were not conducted. Therefore, this study was conducted to characterize the response of Korl:ICR mice to chemicals with the ability to induce gastric ulcer, as well as to compare these effects to those of ICR mice from two other sources. The results indicated that the response to gastric ulcer inducing chemicals in Korl:ICR mice established by the Korea NIFDS were very similar to those of commercial ICR mice.
Many studies have reported similar strategies for the production of gastric ulcer using chemical injection, although the concentration and injection volume of EtOH varied among studies. The phenotypes of gastric ulcer were successfully induced by various combinations of EtOH/HCl concentrations, such as 98% EtOH/150 mM HCl, 60% EtOH/150 mM HCl, 60% EtOH/0.3 M HCl or 60% EtOH/0.45 M HCl in ICR mice [562930]. After induction of gastric ulcer, the expression levels of some inflammatory cytokines such as IL-1β, IL-6, TNF-α, Cox-2 and iNOS were significantly increased by 3 to 4 times compared to the normal group, while the ulcer area, leukocyte infiltration and submucosal edema were enhanced with 1 to 3 times in the gastric ulcer group [34]. Some similar results were observed in the present study, although there were some differences observed upon histological analysis and in cytokine expression. The histopathological structure, including mucosal injury, edema and inflammatory cells, changed significantly with EtOH concentration in all ICR groups. However, the expression level of IL-1β did not coincide with alterations in histological structure. The level of IL-1β was lower in the 90EtOH treated group than in the 50 and 70EtOH treated group. However, more studies are needed to compare various physiological features, including cytokine expression, after the induction of gastric ulcer.
Taken together, we investigated the responses of ICR mice provided from different sources to three concentrations (50%, 70% and 90%) of EtOH in 150 mM HCl with gastric ulcer inducing activity. Three ICR groups, Korl: ICR, A:ICR and B:ICR, show a similar response of body weight, histolopathological structure and inflammation to gastric ulcer inducers. Therefore, our results suggest that Korl:ICR mice can be used as widely as other ICR mice provided from commercial vendors to produce animal models for gastric ulcer.
Acknowledgments
We thank Jin Hyang Hwang, the animal technicians, for directing the Animal Facility and Care at the Laboratory Animal Resources Center. This project was supported by a grant of BIOREIN (Laboratory Animal Bio Resources Initiative) from Ministry of Food and Drug Safety in 2015. Conflict of interests The authors declare that there is no financial conflict of interests to publish these results.
References
1. Viana AF, Fernandes HB, Silva FV, Oliveira IS, Freitas FF, Machado FD, Costa CL, Arcanjo DD, Chaves MH, Oliveira FA, Oliveira RC. Gastroprotective activity of Cenostigma macrophyllum Tul. var acuminata Teles Freire leaves on experimental ulcer models. J Ethnopharmacol. 2013; 150(1):316–323. PMID: 24035848.
2. Yamamoto S, Watabe K, Araki H, Kamada Y, Kato M, Kizu T, Kiso S, Tsutsui S, Tsujii M, Kihara S, Funahashi T, Shimomura I, Hayashi N, Takehara T. Protective role of adiponectin against ethanol-induced gastric injury in mice. Am J Physiol Gastrointest Liver Physiol. 2012; 302(8):G773–G780. PMID: 22323129.

3. Wang J, Zhang T, Zhu L, Ma C, Wang S. Anti-ulcerogenic effect of Zuojin Pill against ethanol-induced acute gastric lesion in animal models. J Ethnopharmacol. 2015; 173:459–467. PMID: 25959443.

4. Chen S, Zhu K, Wang R, Zhao X. Preventive effect of polysaccharides from the large yellow croaker swim bladder on HCl/ethanol induced gastric injury in mice. Exp Ther Med. 2014; 8(1):316–322. PMID: 24944640.

5. Yang Y, Lee J, Rhee MH, Yu T, Baek KS, Sung NY, Kim Y, Yoon K, Kim JH, Kwak YS, Hong S, Kim JH, Cho JY. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015; 39(1):61–68. PMID: 25535478.

6. Byun JS. Protective effects of Coptidis Rhizoma on ethanol-induced gastric ulcer in mice. Korean J Orient Physiol Pathol. 2012; 26(1):67–73.
7. Kim JS, Lim SW. The effects of Sasammaickmoondong-tang against gastric mucosal lesions. J Korean Orient Med. 2003; 24(2):121–137.
8. Adinortey MB, Ansah C, Galyuon I, Nyarko A. In Vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. 2013; 2013:12.
9. Brzozowski T, Konturek PC, Konturek SJ, Kwiecién S, Pajdo R, Brzozowska I, Hahn EG. Involvement of endogenous cholecystokinin and somatostatin in gastroprotection induced by intraduodenal fat. J Clin Gastroenterol. 1998; 27(Suppl 1):S125–S137. PMID: 9872509.

10. Nam DE, Kim OK, Shim TJ, Lee JK, Hwang KT. Inhibitory effects of Chios Mastic Gum on gastric acid secretion by histamine-related pathway in a rat model and primary parietal cells. J Korean Soc Food Sci Nutr. 2014; 43(10):1500–1509.

11. Kazumori H, Ishihara S, Fukuda R, Kinoshita Y. Time-course changes of ECL cell markers in acetic acid-induced gastric ulcers in rats. Aliment Pharmacol Ther. 2002; 16(2):10–19. PMID: 11966519.

12. Liu J, Wang F, Luo H, Liu A, Li K, Li C, Jiang Y. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int Immunopharmacol. 2016; 30:179–187. PMID: 26604089.

13. Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979; 77(3):433–443. PMID: 456839.
14. Oates PJ, Hakkinen JP. Studies on the mechanism of ethanol-induced gastric damage in rats. Gastroenterology. 1998; 94(1):10–21. PMID: 3335281.

15. Ku SK, Seo BI, Park JH, Park GY, Seo YB, Kim JS, Lee HS, Roh SS. Effect of Lonicerae Flos extracts on reflux esophagitis with antioxidant activity. World J Gastroenterol. 2009; 15(38):4799–4805. PMID: 19824114.
16. Laine L, Weinstein WM. Histology of alcoholic hemorrhagic "gastritis": a prospective evaluation. Gastroenterology. 1988; 94(6):1254–1262. PMID: 3258836.

17. Prussin C, Metcalfe DD. 4. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2003; 111(2 suppl):S486–S494. PMID: 12592295.

18. Anderson DC, Kodukula K. Biomarkers in pharmacology and drug discovery. Biochem Pharmacol. 2014; 87(1):172–188. PMID: 24001556.

19. Bueters T, Ploeger BA, Visser SA. The virtue of translational PKPD modeling in drug discovery: selecting the right clinical candidate while sparing animal lives. Drug Discov Today. 2013; 18(17-18):853–862. PMID: 23665277.

21. McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014; 87(1):162–171. PMID: 23954708.

22. Jiminez JA, Uwiera TC, Douglas Inglis G, Uwiera RR. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog. 2015; 7:29. PMID: 26561503.

23. Watanabe K. Prospects for future development in the pharmacology of gastric ulcer models and of gastric acid secretion in experimental animals. Nihon Yakurigaku Zasshi. 1999; 114(3):141–148. PMID: 10553577.
24. Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine. 2nd ed. New York: Academic press;2002.
25. Lynch CJ. The so-called Swiss mouse. Lab Anim Care. 1969; 19(2):214–220. PMID: 4240230.
26. Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005; 37(11):1181–1186. PMID: 16254564.

27. Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993; 27(2):116–123. PMID: 8501892.

28. Lehoczky JA, Cai WW, Douglas JA, Moran JL, Beier DR, Innis JW. Description and genetic mapping of Polypodia: an X-linked dominant mouse mutant with ectopic caudal limbs and other malformations. Mamm Genome. 2006; 17(9):903–913. PMID: 16964440.

29. Jalilzadeh-Amin G, Najarnezhad V, Anassori E, Mostafavi M, Keshipour H. Antiulcer properties of Glycyrrhiza glabra L. extract on experimental models of gastric ulcer in mice. Iran J Pharm Res. 2015; 14(4):1163–1170. PMID: 26664383.
30. Martins JL, Rodrigues OR, da Silva DM, Galdino PM, de Paula JR, Romão W, da Costa HB, Vaz BG, Ghedini PC, Costa EA. Mechanisms involved in the gastroprotective activity of Celtis iguanaea (Jacq.) Sargent on gastric lesions in mice. J Ethnopharmacol. 2014; 155(3):1616–1624. PMID: 25153020.
Figure 1
Stomach lesions of the ICR mice administered ethanol (EtOH)/hydrochloric acid (HCl). (A) Morphological features were observed in the stomachs of ICR mice derived from three different sources after opening along the greater curvature. Arrows indicate the area of hemorrhagic lesions in the inner surface of the stomach. (B) After measurement of the area of visible erosive lesions, the lesion index was calculated using the formula described in the materials and methods. The data shown represent the means±standard deviation (SD) of three replicates.
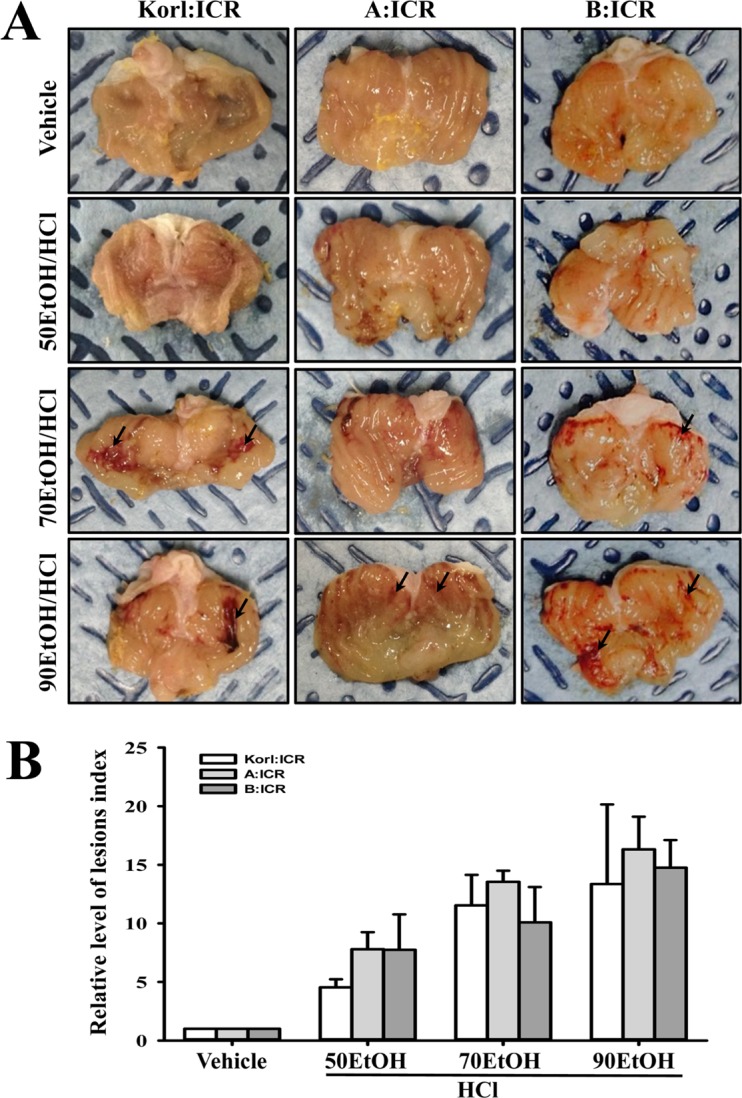
Figure 2
Histopathological analysis of stomach tissue. After the collection of stomachs from the subset group, the histopathological changes in the slide sections of stomach tissue were identified by staining with hematoxylin and eosin followed by observation at 400× magnification. Abbreviations: L, lumen; GP, gastric pits; SC, surface mucous cells.
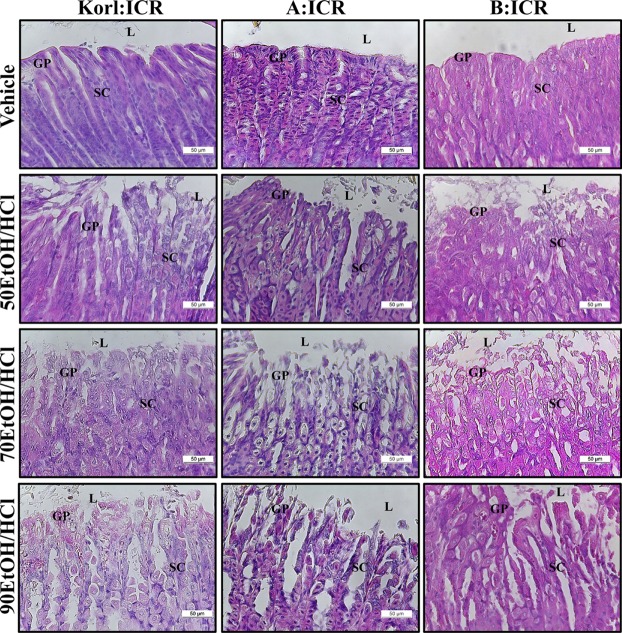
Figure 3
Analysis of mast cells. Infiltration of mast cells in the slide sections of stomach tissue was identified by staining with toluidine blue followed by observation at 400× magnification. Arrows indicate the infiltrated mast cells in the gastric pits of the stomach tissue. The data shown represent the means±standard deviation (SD) of three replicates.
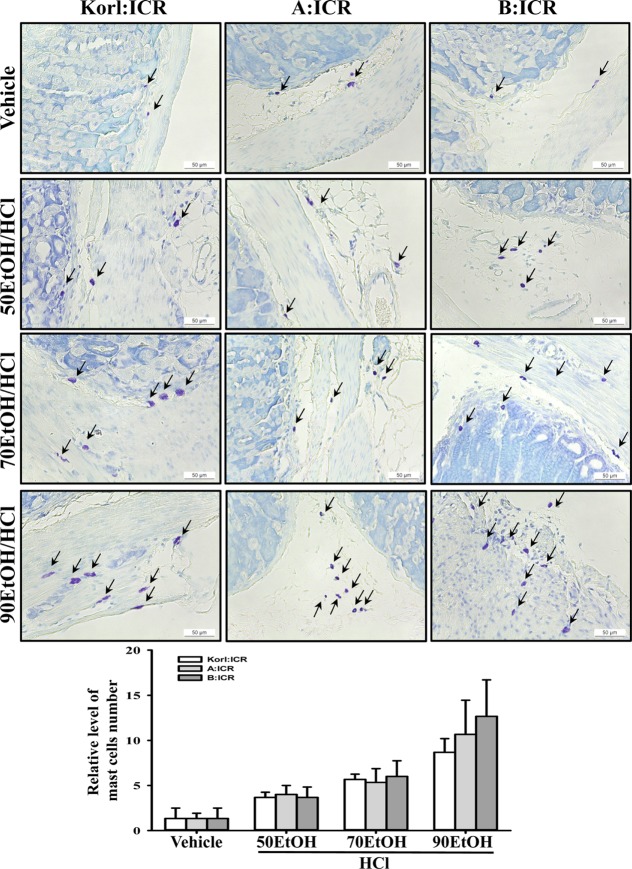
Figure 4
Analysis of cytokine expression in stomach tissues. The levels of IL-1β in the stomach tissue of ICR mice from subset groups were detected by RT-PCR analysis using specific primers. The intensity of each band was determined using an imaging densitometer and the relative level of each protein was calculated based on the intensity of actin transcript as an endogenous control. The data shown represent the means±SD of three replicates. *P<0.05 relative to the Korl:ICR group.
Table 1
Changes on the body weight in ICR mice
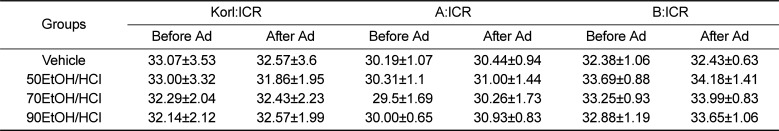
Table 2
Histomorphometrical analysis on the gastric mucosa in EtOH/HCl treated ICR mice




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download