1. Sibal LR, Samson KJ. Nonhuman primates: a critical role in current disease research. ILAR J. 2001; 42(2):74–84. PMID:
11406709.

2. VandeBerg JL, Williams-Blangero S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J Med Primatol. 1997; 26(3):113–119. PMID:
9379477.

3. Dutrillaux B, Viegas-Péquignot E, Dubos C, Masse R. Complete or almost complete analogy of chromosome banding between the baboon (Papio papio) and man. Hum Genet. 1978; 43(1):37–46. PMID:
97206.

4. VandeBerg JL, Williams-Blangero S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J Med Primatol. 1997; 26(3):113–119. PMID:
9379477.

5. Bonfanti U, Lamparelli D, Colombo P, Bernardi C. Hematology and serum chemistry parameters in juvenile cynomolgus monkeys (Macaca fascicularis) of Mauritius origin: comparison between purpose-bred and captured animals. J Med Primatol. 2009; 38(4):228–235. PMID:
19236562.

6. Drevon-Gaillot E, Perron-Lepage MF, Clément C, Burnett R. A review of background findings in cynomolgus monkeys (Macaca fascicularis) from three different geographical origins. Exp Toxicol Pathol. 2006; 58(2-3):77–88. PMID:
16984807.

7. Drevon-Gaillot E, Perron-Lepage MF, Clément C, Burnett R. A review of background findings in cynomolgus monkeys (Macaca fascicularis) from three different geographical origins. Exp Toxicol Pathol. 2006; 58(2-3):77–88. PMID:
16984807.

8. Kimura N, Tanemura K, Nakamura S, Takashima A, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y. Age-related changes of Alzheimer's disease-associated proteins in cynomolgus monkey brains. Biochem Biophys Res Commun. 2003; 310(2):303–311. PMID:
14521910.

9. Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci. 2008; 28(1):3–9. PMID:
18171917.

10. Jayo MJ, Jerome CP, Lees CJ, Rankin SE, Weaver DS. Bone mass in female cynomolgus macaques: a cross-sectional and longitudinal study by age. Calcif Tissue Int. 1994; 54(3):231–236. PMID:
8055372.

11. Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP. Osteoarthritis in cynomolgus macaques. III: Effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res. 1996; 11(9):1209–1217. PMID:
8864894.

12. Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ Jr, Kaplan JR. Old world nonhuman primate models of type 2 diabetes mellitus. ILAR J. 2006; 47(3):259–271. PMID:
16804200.

13. Schuurman HJ, Smith HT. Reference values for clinical chemistry and clinical hematology parameters in cynomolgus monkeys. Xenotransplantation. 2005; 12(1):72–75. PMID:
15598276.

14. Bonfanti U, Lamparelli D, Colombo P, Bernardi C. Hematology and serum chemistry parameters in juvenile cynomolgus monkeys (Macaca fascicularis) of Mauritius origin: comparison between purpose-bred and captured animals. J Med Primatol. 2009; 38(4):228–235. PMID:
19236562.

15. Schuurman HJ, Smith HT. Reference values for clinical chemistry and clinical hematology parameters in cynomolgus monkeys. Xenotransplantation. 2005; 12(1):72–75. PMID:
15598276.

16. Eudey AA. The Crab-Eating Macaque (Macaca fascicularis): Widespread and Rapidly Declining. Primate Conserv. 2008; 23(1):129–132.

17. Xie L, Xu F, Liu S, Ji Y, Zhou Q, Wu Q, Gong W, Cheng K, Li J, Li L, Fang L, Zhou L, Xie P. Age- and sex-based hematological and biochemical parameters for Macaca fascicularis. PLoS One. 2013; 8(6):e64892. PMID:
23762263.

18. Kanthaswamy S, Ng J, Satkoski Trask J, George DA, Kou AJ, Hoffman LN, Doherty TB, Houghton P, Smith DG. The genetic composition of populations of cynomolgus macaques (Macaca fascicularis) used in biomedical research. J Med Primatol. 2013; 42(3):120–131. PMID:
23480663.

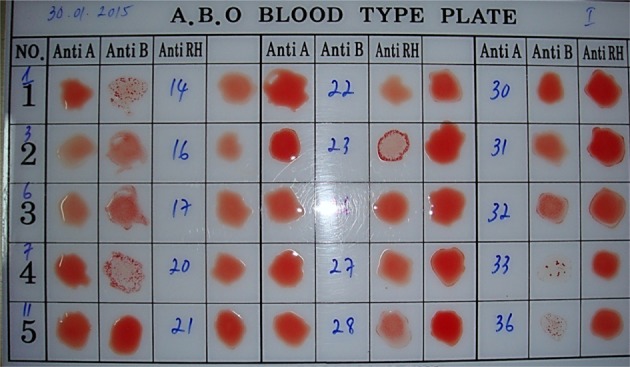
19. Kim TM, Park H, Cho K, Kim JS, Park MK, Choi JY, Park JB, Park WJ, Kim SJ. Comparison of Methods for Determining ABO Blood Type in Cynomolgus Macaques (Macaca fascicularis). J Am Assoc Lab Anim Sci. 2015; 54(3):255–260. PMID:
26045449.
20. Kim C, Kwon M, Lee H, Han S, Heo J, Ha C, et al. Hematologic and Serum Biochemical Variables in Cynomolgus Monkeys. Korean J Lab Anim Sci. 2004.
21. Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995; 59(2):256–262. PMID:
7839449.

22. Aoyama A, Tonsho M, Ng CY, Lee S, Millington T, Nadazdin O, Wain JC, Cosimi AB, Sachs DH, Smith RN, Colvin RB, Kawai T, Madsen JC, Benichou G, Allan JS. Long-term lung transplantation in nonhuman primates. Am J Transplant. 2015; 15(5):1415–1420. PMID:
25772308.

23. Giulietti M, La Torre R, Pace M, Iale E, Patella A, Turillazzi P. Reference blood values of iron metabolism in cynomolgus macaques. Lab Anim Sci. 1991; 41(6):606–608. PMID:
1667207.
24. Sugimoto Y, Hanari K, Narita H, Honjo S. Normal hematologic values in the cynomolgus monkeys aged from 1 to 18 years. Jikken Dobutsu. 1986; 35(4):443–447. PMID:
3803429.

25. Keiji T. CHAPTER 11 - Management of Old World Primates. In : Sonia WC, editor. The Laboratory Primate. London: Academic Press;2005. p. 163–173.
26. Hall RL, Everds NE. Factors affecting the interpretation of canine and nonhuman primate clinical pathology. Toxicol Pathol. 2003; 31(Suppl):6–10. PMID:
12597425.

27. Terao K, Fujimoto K, Cho F, Honjo S. Anti-A and anti-B blood group antibody levels in relation to age in cynomolgus monkeys. Jpn J Med Sci Biol. 1983; 36(5):289–293. PMID:
6668739.

28. Socha WW, Blancher A, Moor-Jankowski J. Red cell polymorphisms in nonhuman primates: a review. J Med Primatol. 1995; 24(4):282–305. PMID:
8750505.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download