Abstract
Intervertebral disc herniation (IVDH) with nucleus pulposus extrusion, traumatic or not, is a devastating clinical condition accompanied by neurological problems. Here we report a cynomolgus macaque suffering from acute and progressive neurological dysfunction by a blunt trauma due to neck collar, an animal handling device. Tetraplegia, urinary incontinence, decreased proprioception, and imperception of pain were shown on physical and neurological examinations. MRI sagittal T2 weighted sequences revealed an extensive protrusion of disc material between C2 and C3 cervical vertebra, and this protrusion resulted in central stenosis of the spinal cord. Histopathologic findings showed a large number of inflammatory cells infiltrated at sites of spinal cord injury (SCI). This case is the first report of compressive cervical SCI caused by IVDH associated with blunt trauma.
Intervertebral disk herniation (IVDH) occurs in both the cervical and thoracolumbar vertebral column and is a common cause of spinal cord injury (SCI) in veterinary species [1]. Hansen described two types of IVDH, classified as Hansen type I or chondroid degeneration and Hansen type II or fibroid degeneration, that can result in IVDH and spinal cord compression [2]. Both types of IVDH can occur in any of the vertebral levels. Meanwhile, Griffiths described another type of IVDH that may also occur in the cervical spine [3]. This type of extrusion occurs when non-degenerated nucleus pulposus extrudes during strenuous exercise or trauma causing spinal cord contusion with little or no residual spinal cord compression [4]. The symptoms may differ depending on the severity, but acute traumatic spinal cord injury (SCI) by vertebral disc herniation or degeneration is a devastating clinical condition in human as well as in animals [56].
Among nonhuman primates (NHPs), cynomolgus monkeys of more than 20 years old and with naturally occurring thoracolumbar IVD degeneration are utilized in orthopedic research [7]. Many SCI cases in NHPs are experimentally induced animal models for the understanding of its pathogenesis and an evaluation of therapeutic regimens in human practices [68910111213]. Until now, there has been no report of SCI from IVDH caused by accidental, rather than deliberate, blunt trauma. In this case report, we describe the clinical and magnetic resonance imaging features of compressive cervical SCI with suspected nucleus pulposus extrusion in a cynomolgus monkey.
A 4-year-old male Cambodia-origin cynomolgus macaque weighing 3.4 kg was housed at an AAALAC-accredited Seoul National University Hospital Nonhuman Primate Center (SNUHNPC) and assigned to type 1 diabetes study approved by the SNUH Institutional Animal Care and Use Committee. For pancreatic islet transplantation study, the monkey was induced to have type 1 diabetes by streptozotocin. Measurement of blood glucose level and administration of insulin were performed twice daily with pole and collar method of restraint [14]. Throughout the experimental period this monkey showed an exceptionally consistent resistive behavior against capture and restraint procedure compared with other monkeys. Unfortunately, whenever this monkey was captured with pole and collar, extensive struggling movement was consistently represented, and this behavior did not improve with time.
At 3 months after diabetes induction, the monkey suddenly presented dull and stiff movement in home cage, while a typical fast and agile movement was remarkably reduced. Eventually, the monkey fell down feebly from a perch of the cage and then was unable to stand up by himself. Urinary incontinence was also observed. On physical examination, the monkey showed a loss of voluntary motor function in thoracic and pelvic limbs, even though normal on joint palpation. In addition, there was no grip strength of the hands or feet. Meanwhile, facial movements including of lips, eyelids, and ears were normal. From hematology and serum biochemistry, there were no significant findings. At the onset of clinical symptoms, a hypoglycemic shock, which occurs commonly in diabetes monkeys because of high dose of exogenous insulin or brittle diabetes, was suspected. However, this was ruled out because the blood glucose level was within the normal range at 73 mg/dL.
On neurological examination, loss of voluntary motor function in all four limbs was observed. The upper motor neuron (UMN) signs showed in thoracic and pelvic limbs. However, no sign involving the head was present. Perception of superficial and deep pain as well as proprioception were decreased in thoracic and pelvic limbs. Moderate hyperesthesia was present on palpation of the cervical area. Based on these findings, we localized the lesion to segment C1-C5.
In conventional radiographs, a distinct abnormality was not found in the cervical region or in other vertebral columns or the musculoskeletal system. However, in a lateral cervical myelogram using Iohexol (Omnipaque, GE, Cork, Ireland), a disappearance of the ventral contrast column was distinctly identified between C1 and C4 (Figure 1). On T2 weighted sagittal and transverse MR images, the cervical disc protrusion was clearly defined between C2 and C3 (Figure 2A, B). This protrusion of disc caused dorsal compression of the spinal cord.
Prophylactic and supportive treatment with cefazolin (25 mg/kg, IV, BID; CKD Pharm., Seoul, Republic of Korea) and methylprednisolone (0.5 mg/kg, IV or IM, SID; Pfizer, NV, Belgium) was initiated with an intravenous fluid therapy (normal saline, 10 mL/kg/hr). In spite of this, the neurologic problems of the monkey were not improved despite long-term treatment. Eventually, the monkey was euthanized after 3 months from the onset. In gross findings, degenerative inflammatory lesion was found between the cervical spinal cord and intervertebral dorsal pedicles. In histopathological findings, the cervical spinal cord was compressed with an extensive area of necrotizing granulomatous inflammation in which a large number of neutrophils and lymphocytes were infiltrated (Figure 3A, B).
Hansen type I IVDH caused by suspected traumatic injury, as described herein, has not been previously reported in nonhuman primates. This case reports that the affected monkey showed acute neurological problems with tetraplegia, and compressive SCI was defined through myelogram, MRI finding, and histopathology.
The vertebral level at which traumatic or degenerative myelopathies occur is diverse and is important in ambulatory function in human as well as veterinary species. In human, the most common site of SCI is the cervical spinal cord (C1-C7) with approximately 55% of injuries [1115], and the number of injuries at this level is increasing [16]. The critical cause of SCI is blunt trauma such as a motor vehicle collision and falls [12]. In dogs, 83.6% of SCI is associated with IVDH, located in the thoracolumbar region between T10 and L7 [17]. Among all dogs with cervical disc herniation, the most commonly affected IVD spaces are represented at C5-C6 and C6-C7 [1819]. In nonhuman primates, the experimentally induced SCI model has been reported, but acute cervical SCI associated IVDH has not been reported.
The affected monkey was trained by using positive and limited negative reinforcement for pole-and-collar moving to the home cage front [14], where he then was restrained. Given time constraints, there was a failure to habituate, resulting in undesirable behavior (that is, the monkey was forcing his neck from the collar) throughout the training period. It seems that repetition of the resistive movement against a collar increased the possibility that a strong force of collision would be delivered to the cervical vertebral column, which in turn made it more likely that the intervertebral disc would become herniated toward the spinal cord.
Images (e.g., plain view, myelogram, and MRI) of the affected monkey, in which the lesion was clinically localized to the C1-C5 spinal cord segment, were taken to include the C1-C5 spinal cord segments in order to investigate the potential underlying cause of the clinical presentation. On T2 weighted sagittal and transverse MR images, unlike the plain and myelogram images, the cervical disc protrusion was clearly defined between C2 and C3. Moreover, the protruded disc excessively compressed toward the spinal cord.
It has been shown with MRI that more severe abnormalities are associated with a more severe neurological status [20]. In other words, hemorrhage, the number of levels of edema, greater degree of cord compression, greater degree of canal compromise, and the severity of soft tissue injury have been shown to be associated with worse neurological outcome in human [2122232425]. This is because blunt SCI in human is characterized by typical pathologic changes including petechial hemorrhages that coalesce into large hemorrhages with time [26]. In particular, compressive SCI associated with IVDH is believed to result in a complex cascade of secondary mechanisms including vascular disturbance, oxidative stress, excitotoxicity, and inflammation [17].
In the current case, although hemorrhagic or edematous lesion was not found in the histopathologic findings carried out in the several weeks post-injury, a disruptive inflammation was revealed between the spinal cord and the intervertebral disc in the autopsy finding. The extruded disc material probably irritated the spinal cord, resulting in inflammation in that area. In a previous study, it was reported that blood-spinal cord barrier disruption and associated innate inflammatory events occurred within hours of SCI and caused neutrophilic diapedesis and microglial activation [27]. Moreover, in the days and weeks after SCI, adaptive immune responses are initiated because of exposure of self-antigens and may cause lymphocytic and mononuclear cell infiltration within injured parenchyma [27].
In conclusion, the underlying cause of clinical symptoms in this case was confirmed through MR images to be a compressive cervical myelopathy caused by acute IVDH. This neurologic deficit was not restored to normal, although initial neurological symptoms were shown to be slightly improved by supportive medical care. It is probable that blunt trauma due to repetitive collision with a neck collar contributed to cervical IVDH and SCI in an untrained cynomolgus monkey.
Acknowledgments
This study was supported by a grant (HI13C0954) of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea.
References
1. Levine GJ, Cook JR, Kerwin SC, Mankin J, Griffin JF, Fosgate GT, Levine JM. Relationships between cerebrospinal fluid characteristics, injury severity, and functional outcome in dogs with and without intervertebral disk herniation. Vet Clin Pathol. 2014; 43(3):437–446. PMID: 24976308.

2. HANSEN HJ. A pathologic-anatomical interpretation of disc degeneration in dogs. Acta Orthop Scand. 1951; 20(4):280–293. PMID: 14894198.

3. Griffiths IR. A syndrome produced by dorso-lateral "explosions" of the cervical intervertebral discs. Vet Rec. 1970; 87(24):737–741. PMID: 5531244.

4. Beltran E, Dennis R, Doyle V, de Stefani A, Holloway A, de Risio L. Clinical and magnetic resonance imaging features of canine compressive cervical myelopathy with suspected hydrated nucleus pulposus extrusion. J Small Anim Pract. 2012; 53(2):101–107. PMID: 22250580.

5. Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014; 52(2):110–116. PMID: 23439068.

6. Piedras MJ, Hernandez-Lain A, Cavada C. Clinical care and evolution of paraplegic monkeys (Macaca mulatta) over fourteen months post-lesion. Neurosci Res. 2011; 69(2):135–143. PMID: 21078348.

7. Nuckley DJ, Kramer PA, Del Rosario A, Fabro N, Baran S, Ching RP. Intervertebral disc degeneration in a naturally occurring primate model: radiographic and biomechanical evidence. J Orthop Res. 2008; 26(9):1283–1288. PMID: 18404651.

8. Black P, Markowitz RS, Cooper V, Mechanic A, Kushner H, Damjanov I, Finkelstein SD, Wachs KC. Models of spinal cord injury: Part 1. Static load technique. Neurosurgery. 1986; 19(5):752–762. PMID: 3785621.
9. Miller AD, Westmoreland SV, Evangelous NR, Graham A, Sledge J, Nesathurai S. Acute traumatic spinal cord injury induces glial activation in the cynomolgus macaque (Macaca fascicularis). J Med Primatol. 2012; 41(3):202–209. PMID: 22620270.

10. Nesathurai S, Graham WA, Mansfield K, Magill D, Sehgal P, Westmoreland SV, Prusty S, Rosene DL, Sledge JB. Model of traumatic spinal cord injury in Macaca fascicularis: similarity of experimental lesions created by epidural catheter to human spinal cord injury. J Med Primatol. 2006; 35(6):401–404. PMID: 17214670.

11. Nout YS, Rosenzweig ES, Brock JH, Strand SC, Moseanko R, Hawbecker S, Zdunowski S, Nielson JL, Roy RR, Courtine G, Ferguson AR, Edgerton VR, Beattie MS, Bresnahan JC, Tuszynski MH. Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury. Neurotherapeutics. 2012; 9(2):380–392. PMID: 22427157.

12. Sledge J, Graham WA, Westmoreland S, Sejdic E, Miller A, Hoggatt A, Nesathurai S. Spinal cord injury models in non human primates: are lesions created by sharp instruments relevant to human injuries? Med Hypotheses. 2013; 81(4):747–748. PMID: 23948598.

13. Darian-Smith C. Monkey models of recovery of voluntary hand movement after spinal cord and dorsal root injury. ILAR J. 2007; 48(4):396–410. PMID: 17712225.

14. McMillan JL, Perlman JE, Galvan A, Wichmann T, Bloomsmith MA. Refining the pole-and-collar method of restraint: emphasizing the use of positive training techniques with rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci. 2014; 53(1):61–68. PMID: 24411781.
15. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001; 26(24 Suppl):S2–S12. PMID: 11805601.

16. DeVivo MJ, Chen Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch Phys Med Rehabil. 2011; 92(3):332–338. PMID: 21353817.

17. Levine JM, Levine GJ, Porter BF, Topp K, Noble-Haeusslein LJ. Naturally occurring disk herniation in dogs: an opportunity for pre-clinical spinal cord injury research. J Neurotrauma. 2011; 28(4):675–688. PMID: 21438715.

18. Ryan TM, Platt SR, Llabres-Diaz FJ, McConnell JF, Adams VJ. Detection of spinal cord compression in dogs with cervical intervertebral disc disease by magnetic resonance imaging. Vet Rec. 2008; 163(1):11–15. PMID: 18603629.

19. Hillman RB, Kengeri SS, Waters DJ. Reevaluation of predictive factors for complete recovery in dogs with nonambulatory tetraparesis secondary to cervical disk herniation. J Am Anim Hosp Assoc. 2009; 45(4):155–163. PMID: 19570897.

20. Bozzo A, Marcoux J, Radhakrishna M, Pelletier J, Goulet B. The role of magnetic resonance imaging in the management of acute spinal cord injury. J Neurotrauma. 2011; 28(8):1401–1411. PMID: 20388006.

21. Dai L, Jia L. Central cord injury complicating acute cervical disc herniation in trauma. Spine (Phila Pa 1976). 2000; 25(3):331–335. PMID: 10703105.

22. Flanders AE, Spettell CM, Friedman DP, Marino RJ, Herbison GJ. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. AJNR Am J Neuroradiol. 1999; 20(5):926–934. PMID: 10369368.
23. Song KJ, Kim GH, Lee KB. The efficacy of the modified classification system of soft tissue injury in extension injury of the lower cervical spine. Spine (Phila Pa 1976). 2008; 33(15):E488–E493. PMID: 18594446.

24. Miyanji F, Furlan JC, Aarabi B, Arnold PM, Fehlings MG. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome--prospective study with 100 consecutive patients. Radiology. 2007; 243(3):820–827. PMID: 17431129.
25. Selden NR, Quint DJ, Patel N, d'Arcy HS, Papadopoulos SM. Emergency magnetic resonance imaging of cervical spinal cord injuries: clinical correlation and prognosis. Neurosurgery. 1999; 44(4):785–792. PMID: 10201304.

26. Kakulas BA. The clinical neuropathology of spinal cord injury. A guide to the future. Paraplegia. 1987; 25(3):212–216. PMID: 3601429.

27. Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008; 209(2):378–388. PMID: 17662717.

Figure 1
Cervical myelogram of the monkey with a disappearance (yellow arrow) of ventral contrast column between C1 and C4.
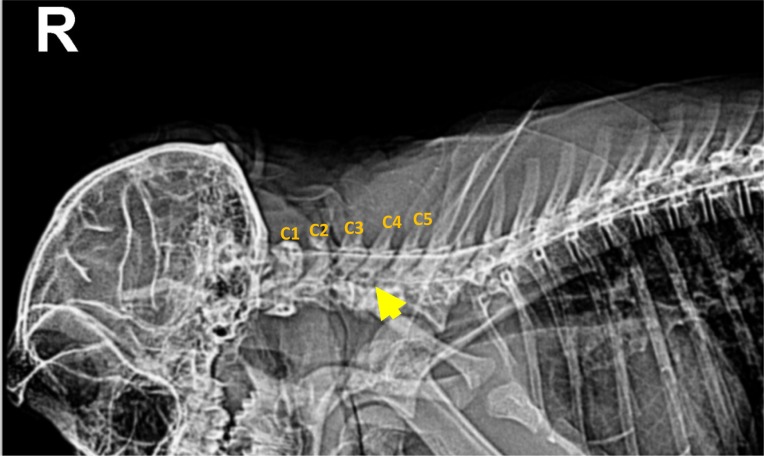
Figure 2
(A) Sagittal T2-weighted image of a suspected extradural compressive material above C2-C3. Extension and narrowing of the affected spinal cord is also evident. (B) Transverse T2-weighted image with the outlined cross-sectional area of the spinal cord at the level of compression.
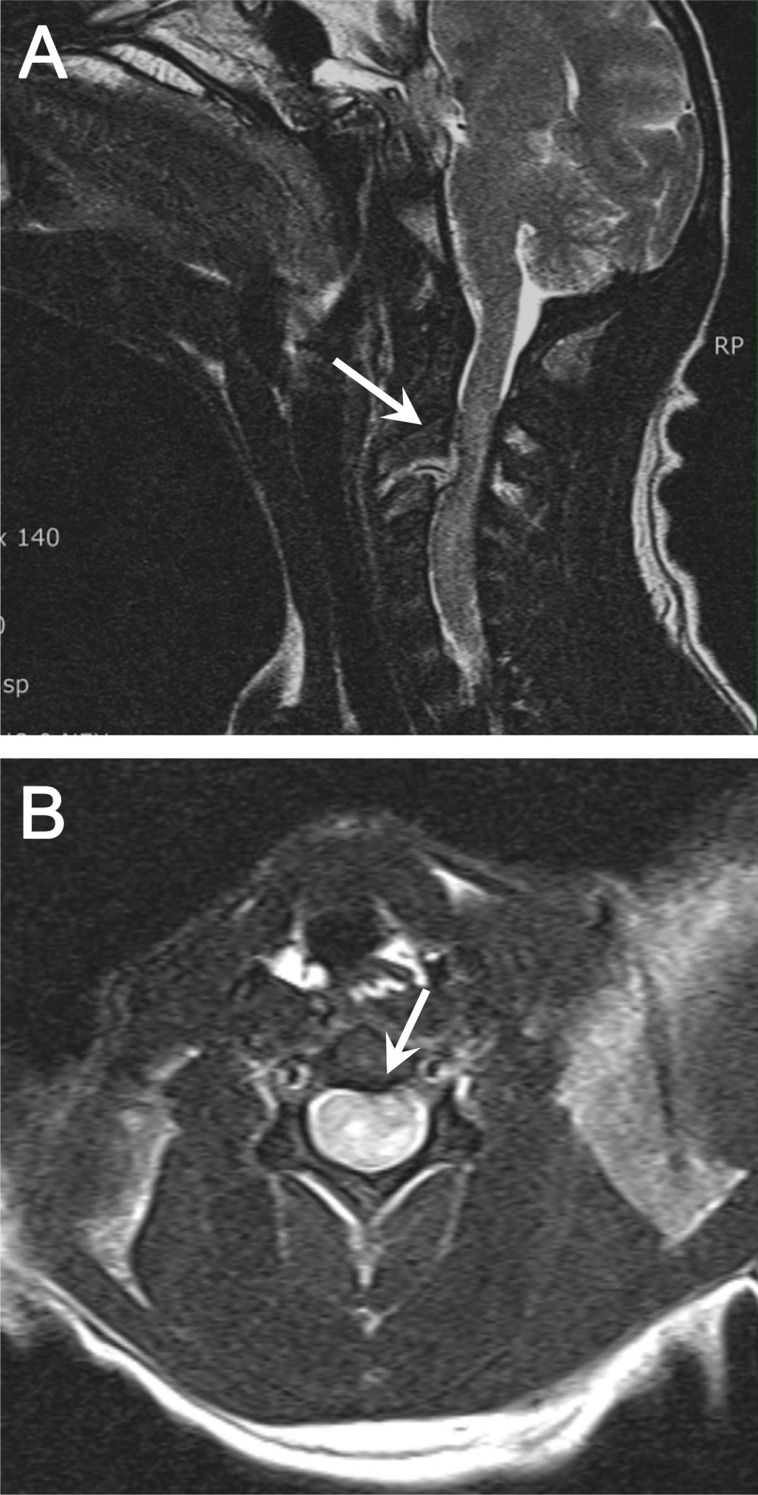
Figure 3
Histopathology of the affected spinal cord (H&E stain). (A) Excessive compression of inflammatory degeneration from the dura mater (DM) to the white mater (WM), (40× magnification, bar=200 µm). (B) A large number of inflammatory cells including neutrophils and lymphocytes are infiltrated (400× magnification, bar=50 µm).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download