Abstract
Anti-atherosclerosis effects of perilla oil were investigated, in comparison with lovastatin, in rabbits fed a high-cholesterol diet (HCD). Hypercholesterolemia was induced in rabbits by feeding the HCD containing 0.5% cholesterol and 1% corn oil, and perilla oil (0.1 or 0.3%) was added to the diet containing 0.5% cholesterol for 10 weeks. HCD greatly increased blood total cholesterol and low-density lipoproteins, and caused thick atheromatous plaques, covering 74% of the aortic wall. Hyper-cholesterolemia also induced lipid accumulation in the liver and kidneys, leading to lipid peroxidation. Perilla oil not only attenuated hypercholesterolemia and atheroma formation, but also reduced fat accumulation and lipid peroxidation in hepatic and renal tissues. The results indicate that perilla oil prevents atherosclerosis and fatty liver by controlling lipid metabolism, and that it could be the first choice oil to improve diet-induced metabolic syndrome.
In modern society, change in the pattern of food consumption increases metabolic syndrome such as obesity, atherosclerosis, hypertension, stroke, diabetes, and cancer [12]. In particular, hyperlipidemia is indicated to be the main cause of health problems in metabolic syndrome [3]. The major risk factors of atherosclerosis and cardiovascular diseases are increased blood levels of total cholesterol (TC) and low-density lipoproteins (LDL), accompanying decrease in high-density lipoproteins (HDL) [45]. It is well known that oxidized LDL (OxLDL) is a triggering molecule of endothelial injury and atheroma formation, whereas HDL is a transporter of cholesterol for metabolism in the liver [6789]. Therefore, it is believed that suppression of blood TC and LDL as well as enhancement of HDL are front-line strategies for the prevention of atherosclerosis [89].
To date, statins has been used to inhibit 3-hydroxy-3-methylglutanyl-coenzyme A (HMG-CoA) reductase, a rate-limiting step enzyme in cholesterol biosynthesis. In addition, statins enhance the activity of LDL receptors on cell membrane, facilitating the metabolism of very low-density lipoproteins (VLDL) remnant and LDL [1011]. In spite of the cholesterol-lowering advantage, however, over-dosage and/or long-term use of statins exert serious adverse-effects like hepatomegaly and renal hypertrophy [1213]. Thus, researches on the novel natural products with minimal adverse-effects to control blood cholesterol level and to improve blood flow are required.
Since the consumption of high-fat diets is the major cause of disturbances in blood flow, selection of fats and oils might be very important. It is well known that unsaturated fatty acids (UFA) properly regulate blood lipid profiles, and thereby improved coronary heart disease [14151617]. Fish oil ω-3 polyunsaturated fatty acids (PUFA) prevented vasoconstriction [18], and suppressed vascular inflammatory response by decreasing production of reactive oxygen species (ROS) [1920]. Notably, α-linolenic acid (ALA), a well-known ω-3 PUFA rich in perilla oil improved insulin sensitivity and hyperlipidemia, and prevented coronary heart disease [2122]. Especially, in recent studies, we demonstrated that perilla oil possessing a low linoleic acid/α-linolenic acid (ω-6/ω-3) ratio not only inhibited platelet aggregation and improved blood flow [23], but also delayed and attenuated brain hemorrhage in stroke-prone spontaneously hypertensive rats (SHR-SP), thereby extending their lifespan [24].
Since PUFA affects both platelets and endothelial cells that play a crucial role in the regulation of thrombosis and haemostasis [25], we investigated the effects of perilla oil on the high-cholesterol diet (HCD)-induced hypercholesterolemia and atherosclerosis.
Perilla oil was obtained from Anydoctor Healthcare Co., Ltd. (Cheonan, Korea). Perilla oil was extracted under a cold-pressed method at 30-48℃, and analyzed with Varian 3800 gas chromatograph (Varian Inc., Walnut Creek, CA, USA) equipped with a Supelcowax 10 fused-silica capillary column (Supelco, Bellefonte, PA, USA). From the fatty acid analysis, it was found that perilla oil contains 72.12% PUFA, 19.1% monounsaturated fatty acids (MUFA), and 8.49% saturated fatty acids (SFA). Especially, among PUFA, 57.47% was ω-3 α-linolenic acid [ALA, 18:2(n-3)] [2324].
Seven-month-old male New Zealand white rabbits (body weight 2.5 kg) were procured from Samtaco Co. (Osan, Korea), and subjected to the experiment after 2-week acclimation to the laboratory environment. The animals were housed in each cage with free access to feed and water under constant environmental conditions (23±2℃ temperature; 45-65% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). All the animal experiments were conducted according to the Standard Operation Procedures, and approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Korea.
Acute dietary hypercholesterolemia was induced by feeding rabbits with a powdered HCD containing 0.5% cholesterol and 1% corn oil for 2 weeks, followed by 0.5% cholesterol for additional 10 weeks during treatment period [262728]. After 2-week induction period, the animals were grouped (n=6/group) according to their blood cholesterol levels to adjust to similar mean values. To assess therapeutic efficacy of perilla oil against hypercholesterolemia and atherosclerosis, the rabbits were fed the HCD containing 0.1 or 0.3% perilla oil during the 10-week period.
Body weights were recorded every week from immediately before starting the experiment. After 16-hour fasting at the end of 2-week hypercholesterolemia induction period and every 2 week during 10-week treatment period, blood sample was collected from auricular artery, and lipid profiles and parameters of hepatic and renal functions were measured in sera using a blood chemistry analyzer (Hitachi-747; Hitachi Korea, Seoul, Korea). The parameters include TC, LDL, HDL, triglycerides (TG), alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), total proteins (TP), glucose, blood urea nitrogen (BUN), and creatinine.
The aortic arch (10 cm) was dissected from the aortic valve of the heart and washed with saline. The sample was dissected in 5 cm long from the orifice of carotid artery, and opened longitudinally. After removing fats and tissues adhering to the adventitia, the aorta was dehydrated with 100% propylene glycol for 10 min, and stained with 0.7% Sudan IV (in propylene glycol) for 10 min [262728]. Then, it was hydrated with 85% propylene glycol for 5 min, washed with distilled water, and photographed and analyzed with Digital Image Analyzer (Image Inside; Focus, Seoul, Korea) for red atheromatous plaques. The extent of lipid accumulation (atherosclerosis index; AI, %) was calculated as the percent Sudan-positive area to the total area of the aortic wall.
After weighing, liver and kidney tissues were frozen at -70℃, and cryosections (10 µm in thickness) were mounted on gelatin-coated slides. The sections were washed with phosphate-buffered saline (PBS, pH6.5) followed by propylene glycol for 2 min, and stained with 0.5% Oil red O for 1 hour. After washing with 85% propylene glycol, the slides were counterstained with Harris' hematoxylin, washed again with distilled water, and observed under a light microscope.
The liver and kidney tissues were dissected on an ice block. The tissues were homogenized in 19 volumes of 10 mM PBS (pH7.4) to make a 5% homogenate at 4℃. Into the tissue homogenate (250 µL), 250 µL sodium dodecyl sulphate (SDS, 8.1% solution) and 500 µL 20% acetic acid (adjusted to pH3.5) were added. After adding 250 µL 2-thiobarbituric acid (TBA, 0.75% solution), the mixture was boiled in a glass tube capped with aluminum foil for 30 min [29]. Samples were cooled on ice, centrifuged at 13,000 g for 10 min, and absorbance of the supernatant was read at 532 nm for the quantification of malondialdehyde (MDA).
The results are presented as means±standard error. The significance of differences of all results was analyzed by one-way analysis of variance followed by the Dunnett's multiple-range test correction, using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set a priority at P<0.05.
During 10-week treatment period, following 2-week hypercholesterolemia-induction period, there were no abnormal symptoms in control and treated rabbits. The rabbits exhibited a gradual increase in their body weights, although the body weight gain of rabbits fed 0.1% perilla oil was slightly higher than that of control animals without statistical significance (P>0.05) (Figure 1).
After 2-week feeding of 0.5% cholesterol and 1% corn oil for hypercholesterolemia induction, blood TC was dramatically elevated to 738-754 mg/dL from 59 mg/dL of normal level. LDL also increased to 237 mg/dL from 19 mg/dL in normal animals. In comparison, there were slight changes in blood HDL (from 30 to 38-50 mg/dL) and TG (from 47 to 29-47 mg/dL). During additional 10-week feeding only 0.5% cholesterol, the TC, LDL, and TG levels doubled, but HDL decreased to 40% of initial concentration (Figure 2). Notably, addition of perilla oil attenuated the increases in TC, LDL, and TG in a concentration-dependent manner, without affecting HDL level. Such effects were also achieved with lovastatin.
A long-term feeding of HCD caused extensive atheromatous plaques covering 74% of the aortic arch and abdominal artery (Figure 3). The degree of atheromatous plaques formation was markedly attenuated by 10-week treatment with perilla oil to 52% and 45% at 0.1% and 0.3% in diet, respectively. Lovastatin (0.002% in diet) also decreased the atheroma area to 31% of HCD group.
In blood biochemical analysis, a long-term hypercholesterolemia significantly reduced TP level, in comparison with slight increases in ALT, AST, and LDH, indicative of hepatic dysfunction (protein synthesis) rather than acute hepatocytic injury (Table 1). ALP, glucose, BUN, and creatinine levels were not affected by hypercholesterolemia. Notably, perilla oil and lovastatin remarkably restored the blood levels of hepatic dysfunction to normal levels.
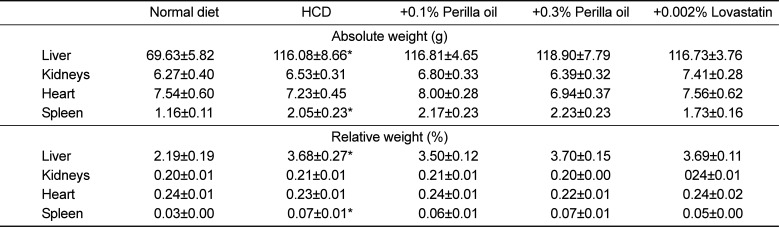
The long-term feeding of HCD significantly increased the weights of the liver and spleen, which were not affected by treatment with perilla oil or lovastatin (Table 2). In microscopic findings, extensive accumulation of lipids were observed in the liver (Figure 4). It is of interest to note that the hepatic steatosis was markedly attenuated by feeding perilla oil in a concentration-dependent manner, in which the effect of 0.3% perilla oil was higher than that of 0.002% lovastatin. Lipid accumulation was also observed focally around the glomerular structures (Figure 5). The glomerular lipid accumulation was remarkably inhibited by perilla oil and lovastatin. Particularly, 0.3% perilla oil fully prevented the renal lipidosis.
In the analysis of lipid peroxidation in the organs showing lipidosis, the MDA concentrations in the liver and kidneys was increased 4 folds and 1.5 folds, respectively, by the long-term HCD feeding (Figure 6). Interestingly, perilla oil (0.1-0.3%) displayed a marked anti-oxidative activity on the MDA formation, comparable with the effect of lovastatin (0.002%).
In the present study, perilla oil substantially inhibited hypercholesterolemia-mediated atheroma formation and lipid accumulation in the liver and kidneys. In addition, perilla oil attenuated the oxidative membrane injury (lipid peroxidation) in the organs showing lipidosis.
Actually, statins such as lovastatin is preferentially prescribed to suppress hepatic cholesterol synthesis, remarkably improving blood lipid profiles [3031]. However, it is well known that over-dosage and long-term administration of the statins cause severe hepatotoxicity, and that low doses could not effectively control the blood cholesterol from diets [1227]. Notably, management of risk factors in preventive mode, rather than therapeutic mode, of the cardiovascular and cerebrovascular diseases is extremely important, because the time to death after outbreak of the diseases is very short [32]. Accordingly, dietary restriction and appropriate choice of fats or oils have been recommended.
While excessive consumption of fats and oils, especially containing high levels of saturated fatty acids (SFA), is known to be harmful for vascular diseases, UFA such as α-linolenic acid (ALA, ω-3) are believed to be beneficial. In fact, it has been reported that perilla oil containing a high level ω-3 PUFA decreased blood TG, TC, and LDL in animals and humans [2033]. Also in human studies, perilla oil not only improved blood HDL and recovered the function of arterial endothelial cells [34], but also reduced blood TG and the risk of cardiac infarction [35]. Also, in the present study, perilla oil containing 72.12% PUFA improved the blood lipid profiles and lipid accumulation in tissues, leading to atherosclerosis and hepatic and renal steatosis.
Notably, in our gas chromatographic analysis of perilla oil, 57.47% was ALA out of 72.12% PUFA. Supportively, it was demonstrated that ALA inhibited platelet activation and arterial thrombus formation [36]. In our recent study, perilla oil markedly suppressed platelet aggregation as well as thrombus formation in the FeCl3-induced endothelial injury model, too [23]. Activated platelets attach to vascular endothelial walls injured during oxidative reaction mediated by OxLDL, aggregate there, and form thrombus and atherosclerosis. Therefore, perilla oil has attracted investigators' attention, because a diet rich in PUFA may be helpful in preventing heart diseases [223738] and blood coagulation [17].
More importantly, it was demonstrated that most of the plant oils with high ω-6/ω-3 fatty acid ratios including canola oil, safflower oil, olive oil, corn oil, and soybean oil, increased hemorrhagic stroke in SHR-SP and shortened their life span, except only perilla oil with a low ω-6/ω-3 fatty acid ratio [15163940]. In our previous study, perilla oil with 0.25 ω-6/ω-3 fatty acid ratio delayed and attenuated brain hemorrhagic stroke and renal lesions in SHR-SP, whereas canola oil with 2.63 ω-6/ω-3 ratio aggravated the lesions, advancing time to death [24].
It was reported that ω-3 PUFA has anti-oxidative and anti-inflammatory activities; it inhibited C-reactive protein in an atherosclerosis model [20] and increased mucosal blood flow by inhibiting leukotriene production in an inflammatory bowel disease model [41]. In the present study, the anti-oxidative activity of perilla oil was also confirmed: i.e., feeding perilla oil markedly suppressed lipid peroxidation in the fatty liver and kidneys following HCD-induced hypercholesterolemia.
To date, statins and anti-coagulants have been used to improve hyperlipidemia and blood flow, and thereby to prevent atherosclerosis and cardiovascular diseases [424344]. In fact, it is well known that platelet activation and aggregation is an initial feature of thrombus formation on the injured arterial endothelium undergoing atherosclerosis [45]. However, the most important triggering factor of oxidative injury of arterial intimal layer, leading to platelet aggregation and clonal expansion of vascular smooth muscle cells, is OxLDL containing a high level of cholesterol. By comparison with harmful effects of various fats and plant oils containing high ω-6/ω-3 fatty acid ratios [3940], it was confirmed that perilla oil, only an oil containing a low ω-6/ω-3 ratio, exerted beneficial effects on hemorrhagic stroke and atherosclerosis in our previous and present studies. Therefore, it is strongly recommended that perilla oil could be the first choice on modern food tables consuming high amount of fats and oils.
Acknowledgments
This work was supported by "Food Functionality Evaluation program" under the Ministry of Agriculture, Food and Rural Affairs and partly Korea Food Research Institute (G2015).
References
1. Huh KB. The present status of nutrition-related diseases and its countermeasures. Korean J Nutr. 1990; 23(3):197–207.
2. Lee HK. Pattern of disease incidence and nutrition in Korea. Korean J Nutr. 1996; 29(4):381–383.
3. Lee YC. Hypercholesterolemia in Korea and nutritional factors. J Korean Soc Lipidol Atheroscler. 1991; 1(1):111–122.
4. Spady DK, Woollett LA, Dietschy JM. Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Annu Rev Nutr. 1993; 13:355–381. PMID: 8369151.

5. Natio HK. Atherogenesis: current topics on etiology and risk factors. Clin Chem. 1995; 41(1):132–133.
6. Bemis CE, Gorlin R, Kemp HG, Herman MV. Progression of coronary artery disease. A clinical arteriographic study. Circulation. 1973; 47(3):455–464. PMID: 4692207.
7. Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971; 74(1):1–12. PMID: 5539274.
8. Jialal I, Devaraj S. The role of oxidized low density lipoprotein in atherogenesis. J Nutr. 1996; 126(4 suppl):1053S–1057S. PMID: 8642431.

9. Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001; 104(4):503–516. PMID: 11239408.
10. Blum A, Simsolo C, Hasin Y. 3-Hydroxy-3-methylglutaryl coenzyme a (HMG-CoA) reductase inhibitors (statins), atherosclerosis and coronary syndromes. Atherosclerosis. 2004; 175(1):1–5. PMID: 15186940.

11. Larsen ML, Illingworth DR. Drug treatment of dyslipoproteinemia. Med Clin North Am. 1994; 78(1):225–245. PMID: 8283933.

12. Cho JH, Lee NJ, Chai HY, Kim TM, Park JH, Kang JK, Kim YB, Hwang SY. Effect of Hwalgidan SJ-101 on atherosclerosis in hypercholesterolemic rabbits. Lab Anim Res. 2005; 21(2):149–157.
13. Hong SH, Chai HY, Kim TM, Lee NJ, Kim DK, Cho JH, Park JH, Kim YB, Kang JK, Hwang SY. Therapeutic Effects of Mulberry Root-Bark (Mori radicis Cortex) Ethanol Extract on Atherosclerosis in Hypercholesterolemic Rabbits. Lab Anim Res. 2005; 21(3):273–279.
14. Kim HK, Choi S, Choi H. Suppression of hepatic fatty acid synthase by feeding alpha-linolenic acid rich perilla oil lowers plasma triacylglycerol level in rats. J Nutr Biochem. 2004; 15(8):485–492. PMID: 15302084.
15. Huang MZ, Watanabe S, Kobayashi T, Nagatsu A, Sakakibara J, Okuyama H. Unusual effects of some vegetable oils on the survival time of stroke-prone spontaneously hypertensive rats. Lipids. 1997; 32(7):745–751. PMID: 9252963.

16. Okuyama H, Yamada K, Miyazawa D, Yasui Y, Ohara N. Dietary lipids impacts on healthy ageing. Lipids. 2007; 42(9):821–825. PMID: 17546469.

17. Lanzmann-Petithory D. Alpha-linolenic acid and cardiovascular diseases. J Nutr Health Aging. 2001; 5(3):179–183. PMID: 11458289.
18. Vanschoonbeek K, de Maat MP, Heemskerk JW. Fish oil consumption and reduction of arterial disease. J Nutr. 2003; 133(3):657–660. PMID: 12612132.

19. De Caterina R, Cybulsky MA, Clinton SK, Gimbrone MA Jr, Libby P. Omega-3 fatty acids and endothelial leukocyte adhesion molecules. Prostaglandins Leukot Essent Fatty Acids. 1995; 52(2-3):191–195. PMID: 7540306.

20. Zhang L, Geng Y, Yin M, Mao L, Zhang S, Pan J. Low omega-6/omega-3 polyunsaturated fatty acid ratios reduce hepatic C-reactive protein expression in apolipoprotein E-null mice. Nutrition. 2010; 26(7-8):829–834. PMID: 20004083.
21. Griffin MD, Sanders TA, Davies IG, Morgan LM, Millward DJ, Lewis F, Slaughter S, Cooper JA, Miller GJ, Griffin BA. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45-70 y: the OPTILIP Study. Am J Clin Nutr. 2006; 84(6):1290–1298. PMID: 17158408.

22. De Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994; 343(8911):1454–1459. PMID: 7911176.

23. Jang JY, Kim TS, Cai J, Kim J, Kim Y, Shin K, Kim KS, Lee SP, Kang MH, Choi EK, Rhee MH, Kim YB. Perilla oil improves blood flow through inhibition of platelet aggregation and thrombus formation. Lab Anim Res. 2014; 30(1):21–27. PMID: 24707301.

24. Cai J, Jang JY, Kim J, Shin K, Kim KS, Park D, Kim TS, Lee SP, Ahn B, Choi EK, Lee J, Kim YB. Comparative effects of plant oils on the cerebral hemorrhage in stroke-prone spontaneously hypertensive rats. Nutr Neurosci. 2016; 19(7):318–326. PMID: 24856006.

25. Heemskerk JW, Vossen RC, van Dam-Mieras MC. Polyunsaturated fatty acids and function of platelets and endothelial cells. Curr Opin Lipidol. 1996; 7(1):24–29. PMID: 8925184.

26. Cheong SH, Kim MY, Sok DE, Hwang SY, Kim JH, Kim HR, Lee JH, Kim YB, Kim MR. Spirulina prevents atherosclerosis by reducing hypercholesterolemia in rabbits fed a high-cholesterol diet. J Nutr Sci Vitaminol (Tokyo). 2010; 56(1):34–40. PMID: 20354344.

27. Park D, Kyung J, Kim D, Hwang SY, Choi EK, Kim YB. Anti-hypercholesterolemic and anti-atherosclerotic effects of polarized-light therapy in rabbits fed a high-cholesterol diet. Lab Anim Res. 2012; 28(1):39–46. PMID: 22474473.

28. Jang JY, Kim J, Cai J, Kim Y, Shin K, Kim TS, Lee SP, Park SK, Choi EK, Kim YB. An ethanolic extract of Angelica gigas improves atherosclerosis by inhibiting vascular smooth muscle cell proliferation. Lab Anim Res. 2014; 30(2):84–89. PMID: 24999363.
29. Kwon SC, Kim YB. Antioxidative and aldose reductase-inhibitory effects of a fermentation filtrate of Rubus coreanus. Lab Anim Res. 2011; 27(4):365–368. PMID: 22232649.
30. McClelland GA, Stubbs RJ, Fix JA, Pogany SA, Zentner GM. Enhancement of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor efficacy through administration of a controlled-porosity osmotic pump dosage form. Pharm Res. 1991; 8(7):873–876. PMID: 1924137.
31. Yim JE, Choue RW, Kim YS. Effects of dietary counceling and HMG-CoA reductase inhibitor treatment on serum lipid levels in hyperlipidemic patients. Korean Nutr Soc. 1998; 8(1):61–76.
32. Blaha MJ, Bansal S, Rouf R, Golden SH, Blumenthal RS, Defilippis AP. A practical "ABCDE" approach to the metabolic syndrome. Mayo Clin Proc. 2008; 83(3):932–941. PMID: 18674478.

33. Kurowska EM, Dresser GK, Deutsch L, Vachon D, Khalil W. Bioavailability of omega-3 essential fatty acids from perilla seed oil. Prostaglandins Leukot Essent Fatty Acids. 2003; 68(3):207–212. PMID: 12591004.

34. Wei M, Xiong P, Zhang L, Fei M, Chen A, Li F. Perilla oil and exercise decrease expressions of tumor necrosis factor-alpha, plasminogen activator inhibitor-1 and highly sensitive C-reactive protein in patients with hyperlipidemia. J Tradit Chin Med. 2013; 33(2):170–175. PMID: 23789212.
35. Eussen SR, Geleijnse JM, Giltay EJ, Rompelberg CJ, Klungel OH, Kromhout D. Effects of n-3 fatty acids on major cardiovascular events in statin users and non-users with a history of myocardial infarction. Eur Heart J. 2012; 33(13):1582–1588. PMID: 22301766.

36. Holy EW, Forestier M, Richter EK, Akhmedov A, Leiber F, Camici GG, Mocharla P, Luscher TF, Beer JH, Tanner FC. Dietary α-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler Thromb Vasc Biol. 2011; 31(8):1772–1780. PMID: 21571683.

37. Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ. 1996; 313(7049):84–90. PMID: 8688759.

38. Hu FB, Stampfer MJ, Manson JE, Rimm EB, Wolk A, Colditz GA, Hennekens CH, Willett WC. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. 1999; 69(5):890–897. PMID: 10232627.
39. Huang MZ, Naito Y, Watanabe S, Kobayashi T, Kanai H, Nagai H, Okuyama H. Effect of rapeseed and dietary oils on the mean survival time of stroke-prone spontaneously hypertensive rats. Biol Pharm Bull. 1996; 19(4):554–557. PMID: 8860957.

40. Ratnayake S, Lewandowski P. Rapid bioassay-guided screening of toxic substances in vegetable oils that shorten the life of SHRSP rats. Lipids Health Dis. 2010; 9:13. PMID: 20122175.

41. Shimizu T, Igarashi J, Ohtuka Y, Oguchi S, Kaneko K, Yamashiro Y. Effects of n-3 polyunsaturated fatty acids and vitamin E on colonic mucosal leukotriene generation, lipid peroxidation, and microcirculation in rats with experimental colitis. Digestion. 2001; 63(1):49–54. PMID: 11173900.

42. Zhao G, Zang SY, Jiang ZH, Chen YY, Ji XH, Lu BF, Wu JH, Qin GW, Guo LH. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J Nutr Biochem. 2011; 22(10):929–936. PMID: 21190830.

43. Pan CH, Tsai CH, Lin WH, Chen GY, Wu CH. Ethanolic Extract of Vitis thunbergii Exhibits Lipid Lowering Properties via Modulation of the AMPK-ACC Pathway in Hypercholesterolemic Rabbits. Evid Based Complement Alternat Med. 2012; 2012:436786. PMID: 22536284.
44. Sylvester KW, Cheng JW, Mehra MR. Esomeprazole and aspirin fixed combination for the prevention of cardiovascular events. Vasc Health Risk Manag. 2013; 9:245–254. PMID: 23696706.
45. Fintel DJ. Oral antiplatelet therapy for atherothrombotic disease: overview of current and emerging treatment options. Vasc Health Risk Manag. 2012; 8:77–89. PMID: 22393298.

Figure 1
Change in body weights of hypercholesterolemic rabbits fed a high-cholesterol diet (HCD) containing perilla oil or lovastatin for 10 weeks. ○, Normal diet; ●, HCD alone; ▼, HCD+0.1% perilla oil; ■, HCD+0.3% perilla oil; ◆, HCD+ 0.002% lovastatin.
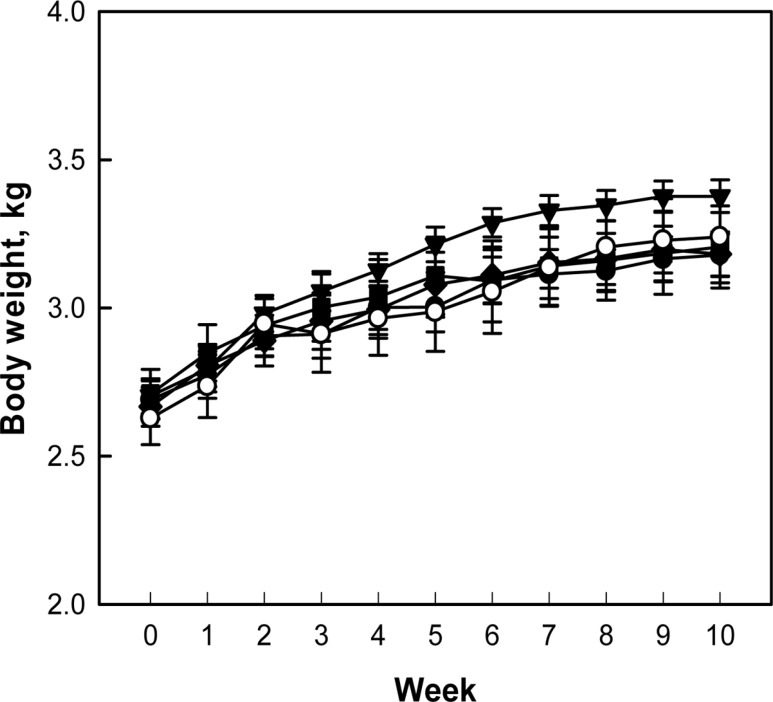
Figure 2
Effects of perilla oil and lovastatin on the blood total cholesterol (TC), low-density lipoproteins (LDL), high-density lipoproteins (HDL), and triglycerides (TG) of high-cholesterol diet (HCD)-fed rabbits (% of initial concentration). ○, Normal diet; ●, HCD alone; ▼, HCD+0.1% perilla oil; ■, HCD+0.3% perilla oil; ◆, HCD+0.002% lovastatin. *Significantly different from normal, P<0.05. #Significantly different from HCD alone, P<0.05.
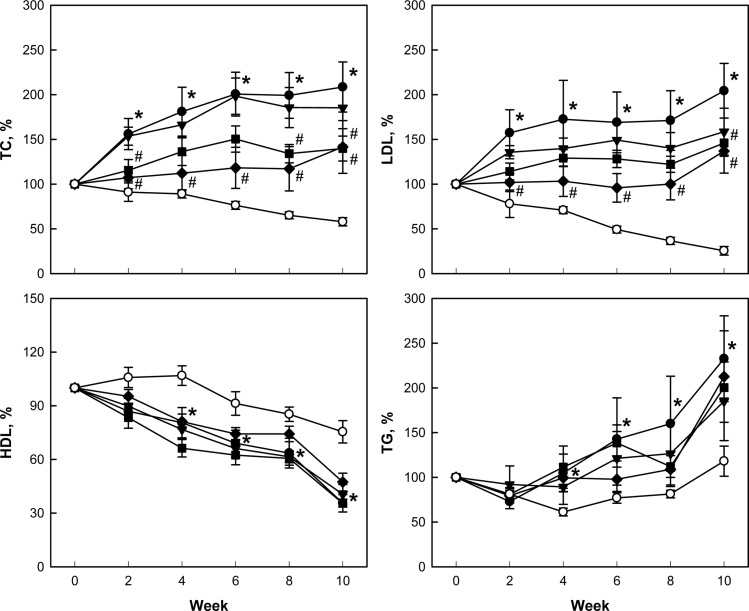
Figure 3
Findings of atheroma of rabbits fed a high-cholesterol diet (HCD) containing perilla oil or lovastatin for 10 weeks. *Significantly different from normal diet (ND), P<0.05. #Significantly different from HCD alone, P<0.05.
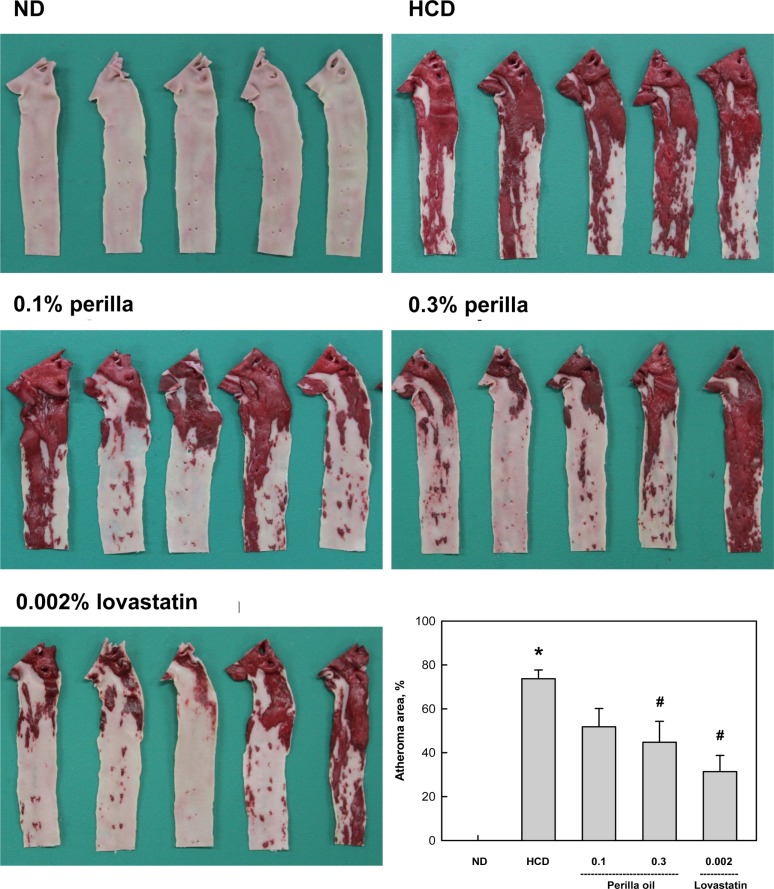
Figure 4
Representative microscopic findings of the liver of rabbits fed a high-cholesterol diet (HCD) containing perilla oil or lovastatin for 10 weeks. ND, normal diet.
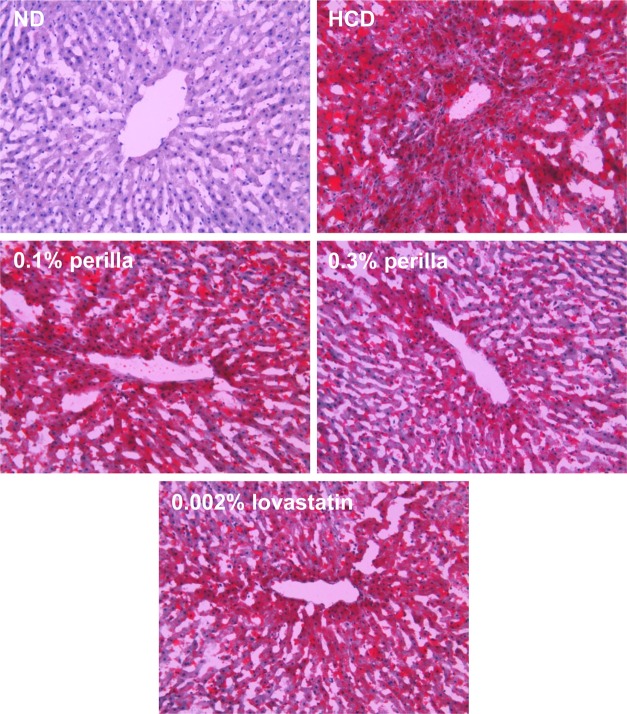
Figure 5
Representative microscopic findings of the kidney of rabbits fed a high-cholesterol diet (HCD) containing perilla oil or lovastatin for 10 weeks. ND, normal diet.
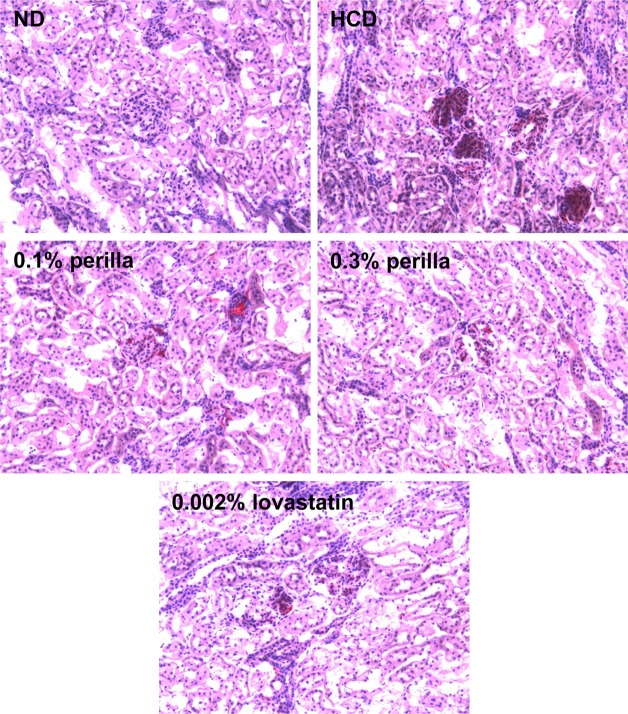
Figure 6
Effects of perilla oil (0.1 or 0.3%) and lovastatin (0.002%) on the lipid peroxidation of liver (A) and kidney (B) tissues of high-cholesterol diet (HCD)-fed rabbits. ND, normal diet.
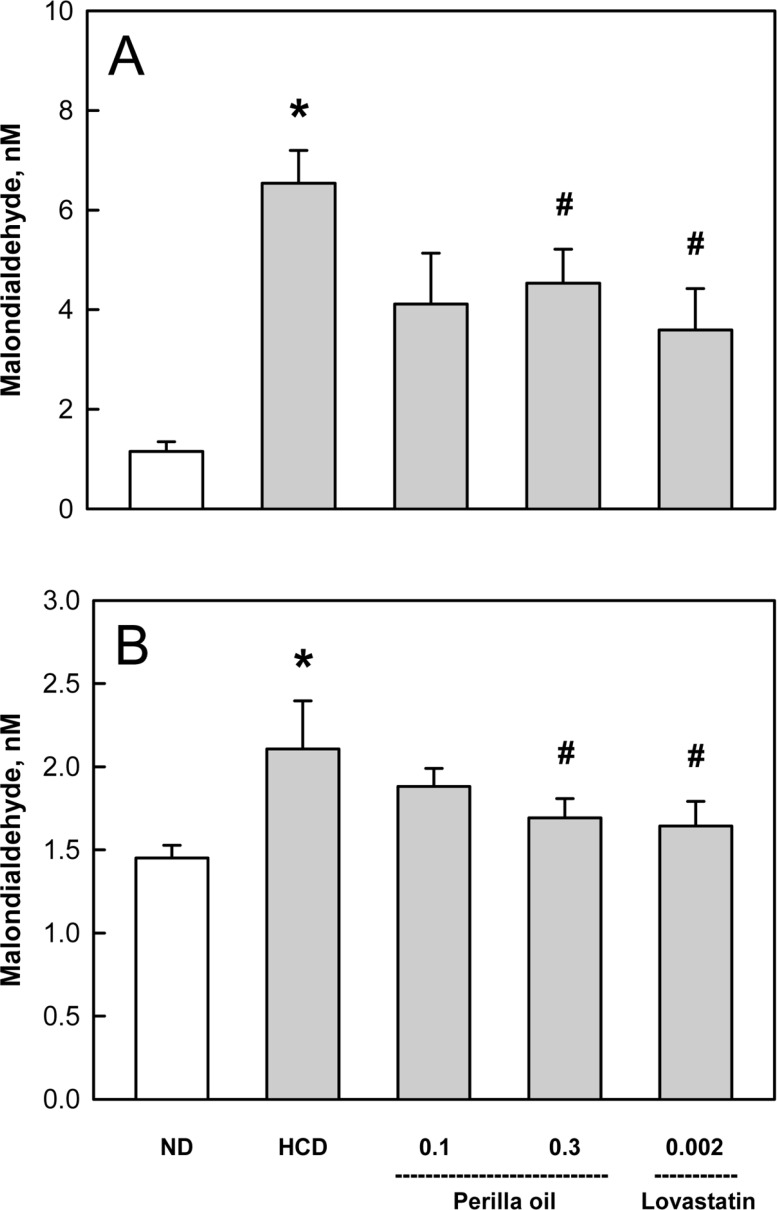
Table 1
Effects of perilla oil and lovastatin on the blood biochemistry of high-cholesterol diet (HCD)-fed rabbits
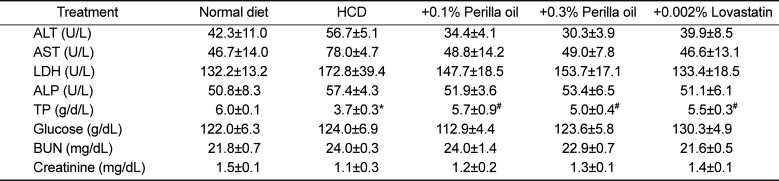
Table 2
Effects of perilla oil and lovastatin on the organ weights of high-cholesterol diet (HCD)-fed rabbits




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download