This article has been
cited by other articles in ScienceCentral.
Abstract
The approach and novelty of this scientific work was to formulate the appropriate Streptozotocin (STZ) and Alloxan dosage in different routes of administration to imply minimum mortality rate and high incidence of diabetes mellitus (DM) in the rat experiment model. Rats were randomly divided into STZ, Alloxan and control groups. 1-Alloxan group was divided into two subgroups: intraperitoneal (ip) subgroups which received a single dose of, 140, 120, 100 and 80 mg/kg; and the subcutaneous (sc) subgroups which received a single dose of, 120, 110, 100, 90, and 80 mg/kg. 2-STZ group was divided into four subgroups of ip route. The ip subgroup which received intraperitoneally a single dose of, 30, 35, 40 and 50 mg/kg. 3-The control group: This group received solo distilled water. The injection day was considered as the day zero. Blood glucose levels and mortality rate were recorded. Subsequently, 30 days after, the logistic regression modeling was used to evaluate the effect of the explanatory variables, the dose levels, and route approaches, on the probability of DM incidence, and mortality. According to the statistical logistic analysis for Alloxan, it is concluded that the minimum dosage needed to induce DM was 120 mg/kg by sc method (probability 0.712). In addition, the logistic analysis for STZ showed that the optimal dose-level for STZ was 40 mg/kg with ip with approximate induction of DM probability 0.764. Based on the data, male Wistar rats in which received a single dosage of Alloxan by sc injection at dose of 120 mg/kg showed the most desirable result of induction of type I DM; furthermore, those in which received STZ by ip injection at the dose of 40 mg/kg developed a persistent and optimal DM state characterized by high rate of DM induction and low- level of mortality.
Go to :

Keywords: Type one diabetes mellitus, alloxan, streptozotocin, intraperitoneal route, subcutaneous route, rate of diabetes induction
Diabetes mellitus (DM) is a general term used for metabolic disorders which are characterized by the increase in the blood glucose (hyperglycemia), lipids and glycosuria level [
1]. In 2000, the prevalence rate for DM was approximately 177 million people worldwide; furthermore, it is estimated by 2025 that 300 million people get affected by DM [
2].
As reported in different scientific studies, there are quite different methods for studding DM and understanding the pathogenesis, complications, genetic, and environmental influences [
3].
The rat animal models are quite suitable for scientific studies in DM; therefore, they are widely used to produce an experimental model for diabetic researches [
4]. To impel animal models for DM, there are many ways including: by chemical induction of DM [
5], by oral glucose loading [
6], by Ferric nitrilotriacetate, by surgical model of DM, and by genetic mutations [
7]. Among them, the chemical agent–induced DM in laboratory animals are the best choose; moreover, the result would be obtained rapidity and simplistic way to produce an experimental model whose value in the elucidation of causal relationship related to human DM [
8]. The induction of DM by the chemical agents make the body changes as same as DM [
9].
Comparing to the entire schemes, the most alluring methods to induce DM are by using Alloxan and Streptozotocin (STZ) [
4]. They are both cytotoxic glucose analogues. Although, the mechanism of the action for STZ and Alloxan are quite different, the mechanisms for selectivity of the beta cells are similar to each other [
56].
STZ and Alloxan, have mostly been used for induction of experimental model for type I DM in animals such as rabbits, rats, mice, and dogs. The mammals develop type I DM when they receive a varying dose of Alloxan ranging from 80 to 140 mg/kg and the dosage which is needed when STZ is used ranges from 45 to 70 [
10]. The dose levels which are required to induce type I DM depends closely to the animal species and the route of administration [
101112].
At lower doses, STZ -and Alloxan- induced DM were not stable, since spontaneous recovery occurred. This phenomenon led to a significant decrease in the incidence rate of experimental model of DM in animals and; in addition, when higher dosages were used, the mortality rate significantly increased [
13]. Precisely, for long-term studies on pathological changes related to DM, researchers require a stable model of experimentally induced type I DM.
Therefore, in this scientific work, the purpose was to realize a proper dosage and effective route of administration for the mentioned diabetogenic chemical agents.
To the best of our knowledge, there has not been any statistical data in which advanced statistical models, such as logistic models was used to formulate the appropriate STZ and Alloxan dosage for various injection routes and for minimizing mortality rate in these settings. The aim of the present scientific study was to introduce the appropriate dose and route of administration for STZ and Alloxan chemical compounds; therefore, it could be possible to produce an optimal experimental model for type I DM with the least mortality rate in male Wister rats.
Materials and Methods
The adult male Wister rats with 4 months of age in which weighted approximately 250±10 g were used in the study. At first all animals were obtained from Pasteur Institute of Iran, secondly they transferred to the animal laboratory, thirdly housed under a 12 hr. light (7 a.m.-7 p.m.)/dark cycle, and fourthly they were feed with a standard laboratory diet and water ad libitum. They were acclimatized in conventional laboratory conditions for 14 days prior to the start of the experiments. All procedures were approved by the Medical Ethics Committee at Shahid Beheshti University of Medical Sciences, Tehran, Iran (protocol no 1393-1-91-500).
Animal grouping
Rats were randomly divided into two experimental groups (the Alloxan, and STZ groups) and one control group. The details of each group, containing: dose levels, the number of cases at each group and the route of administration approaches, are as follows:
Induction of type I DM with Alloxan
Alloxan was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The rats were randomly divided into groups in which received the chemical compounds by intraperitoneal (ip) and subcutaneous (sc). The ip rat groups received a single dosage of 140 mg/kg (number of animals (n=21), 120 mg/kg (n=20), 100 mg/kg (n=19), and 80 mg/kg (n=22)). The sc groups received a single dosage of, 120 mg/kg (n=20), 110 mg/kg (n=23), 100 mg/kg (n=20), 90 mg/kg (n=19), and 80 mg/kg (n=20).
Induction of type I DM with STZ
STZ was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The rats were randomly divided into ip rat groups. The ip groups received intraperitoneally a single dosage of, 30 mg/kg (n=16), 35 mg/kg (n=20), 40 mg/kg (n=19), and 50 mg/kg (n=21).
The control group received an equivalent volume (100 microliter/100 g) of distilled water.
General considerations
All injections were performed under general anesthesia by 50 mg/kg ketamine hydrochloride injected intramuscularly along with 5 mg/kg diazepam. The entire populations were fasted for 12 h before the injection of the chemical compound. The total injection time for each rat was about 7 min. After the administration of the chemical compounds, rats were monitored for changes in their blood glucose levels every seven days for the period of a month by using a glucometer (Accu-Chek, Roche diagnostics India Pvt. Ltd., Mumbai, India). One week after drug administration the blood glucose level for rats were checked. Eventually, after a month, the blood glucose level, the mortality rate, and the rate of diabetic rats were recorded; furthermore, the mentioned data were compared in the three study groups.
Statistical analysis
In the current investigation, a statistical model for the optimal dose level of Alloxan and STZ were recognized in order to induce DM in Wistar male rats; in addition, in the present experiment, different dosage of Alloxan and STZ were separately injected to the special number of rats and diabetic rats were recognized, we used an explanatory variable (dose- level) and a fixed factor for injection method; ip and sc. The fixed factor was transformed to a dummy variable in order to account the injection effect. Therefore, the explanatory variables were "dose- level" and the "route method". The response variable was the number of diabetic rats. In addition, the logistic regression modeling was used to evaluate the effect of the explanatory variables on the probability of infection. The model selection criterion in this application was Pearson chi-squared statistics, in which was appeared from the output of statistical software. Furthermore, SPSS 21 was utilized in this work. A 0.05 level was adopted for any significant difference.
Go to :

Results
Table one shows the Alloxan data and their corresponding estimated probabilities from the logistic analysis. The Pearson goodness-of-fit test led to chi-square value 2.903 with 5 degrees of freedom and significant level 0.715. Therefore, the assumed model fits quite proper. The corresponding model parameter estimates showed a significant effect of the explanatory variables and their interaction. Base on
Table 1, the maximum diabetic rats were belong to dose-level 120 mg/kg by sc method. It is essential to mention the dose level more than 120 mg/kg decreased the probability for making rats diabetic; in addition, increased mortality rate in the models up to 18±9%.
Figure 1 depicts the results.
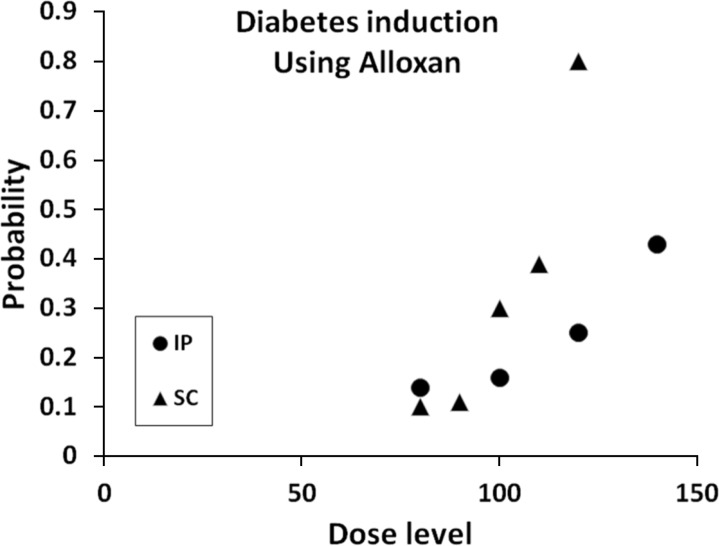 | Figure 1The figure illustrates the estimated probability for induction of type I DM based on Alloxan data, the logistic model, fitted separately for ip (circle symbol) and SC (triangle symbol) methods. Pursuant to the analysis, the two methods showed an increase trend for the probabilities of induction of DM over various dose levels. However, the SC method for Alloxan depicts a larger slop and hence a higher probability of induction for DM at 120mg/kg. Therefore, the more desirable choice would be the mentioned Alloxan level and SC method to induce DM in mammals. It is imperative to note, that the figure does not imply that by increasing the Alloxan dose level, more than 140mg/kg, the probability of induction of DM may also get increased, because beyond this critical level the mortality rate starts to increase rapidly.
|
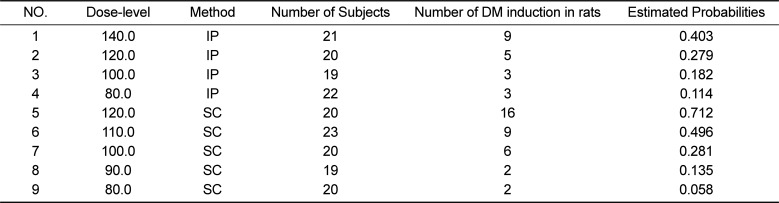
Table 1
This table shows various dose levels, administration methods, the number of subjects, the number of DM induction in rats, and the estimated probability for DM induction by using logistic regression
|
NO. |
Dose-level |
Method |
Number of Subjects |
Number of DM induction in rats |
Estimated Probabilities |
|
1 |
140.0 |
IP |
21 |
9 |
0.403 |
|
2 |
120.0 |
IP |
20 |
5 |
0.279 |
|
3 |
100.0 |
IP |
19 |
3 |
0.182 |
|
4 |
80.0 |
IP |
22 |
3 |
0.114 |
|
5 |
120.0 |
SC |
20 |
16 |
0.712 |
|
6 |
110.0 |
SC |
23 |
9 |
0.496 |
|
7 |
100.0 |
SC |
20 |
6 |
0.281 |
|
8 |
90.0 |
SC |
19 |
2 |
0.135 |
|
9 |
80.0 |
SC |
20 |
2 |
0.058 |

The data for the usage of STZ was statistically analyzed. A quadratic logistic model was selected as a suitable model. The corresponding Pearson goodness-of-fit test showed a chi-square value 0.192 with one degree of freedom and significant level 0.661 which implies a well fit. Moreover, the corresponding model parameter estimates showed a significant effect of the explanatory variables. As stated in
Table 2, it was presented that the optimal dose-level for STZ was 40mg/kg with approximate induction of type I DM probability 0.764, the data can be found in
Table 2. It is important to note that the dose-level 50 mg/kg decreased the probability for induction of type I DM; in addition, caused the decrease in mortality rate up to 42±11%.
Figure 2 depicts the results.
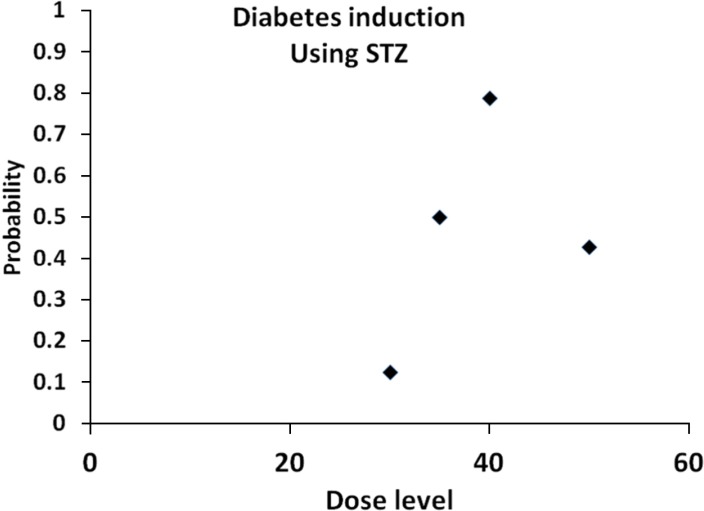 | Figure 2Figure 2 illustrates the estimated probability data for induction of type I DM by STZ in which logistic model was used. As seen the probabilities of induction of DM first increases up to dose level 40mg/kg and then starts to decreases for higher levels. Therefore, we found that the dose level 40mg/kg with STZ by ip method was the best method to induce DM type É in the current study.
|
Table 2
It shows the data scrutiny for STZ including: dose level, the number of subjects, the number of DM induction, and the estimated probability of induction of type one DM by using logistic regression fit

|
NO. |
Dose-level |
Number of Subjects |
Number of induced diabetic rats |
Estimated Probabilities |
|
1 |
30.0 |
16 |
2 |
0.110 |
|
2 |
35.0 |
20 |
10 |
0.532 |
|
3 |
40.0 |
19 |
15 |
0.764 |
|
4 |
50.0 |
21 |
9 |
0.432 |

Go to :

Discussion
The use of experimental induced DM models by chemical agents, such as Alloxan and STZ, is one of the easiest and convenient methods for screening and studying new drugs and new therapeutic modalities [
14].
According to the reports, the selection of an appropriate dose and route for administration are quite important issue [
1214]. Remarkably, in this experiment, it was presented an appropriate dose which is needed for the usage of Alloxan and STZ; in addition, the most proper routes in which type I DM can get induced in the animal models.
In this work, it was investigated the effects of diabetogenic doses of Alloxan range from 80 to 140 mg/kg (80,100,120, and 140) as a single dose by the routes of SC and ip administration; in addition, STZ doses 30, 35, 40, and 50 mg/kg by the route of ip administration on rate of DM induction and mortality in rats. The results showed that the rates of animal survival and induction of DM were more in rats in which received Alloxan at dose of 120 mg/kg by sc and 140 mg/kg ip route than the other dosage groups. Besides, the results indicated that the administration of Alloxan with the dose of 120 mg/kg through sc had optimal results for inducing type I DM; compare to the dose 140 mg/kg and routes of ip administrations. In the other words, the final finding showed that the route of administration was the principal factor for induction of type I DM in rats.
This is similar with the Federiuk, et al. works [
5]. They tried to find an optimal route of administration of the diabetogenic drug, methods of reducing drug toxicities and mortality in rats. They reported that ip administration of the Alloxan at a single high dose (200 mg/kg ip) was the best treatment and led to 70% incidence of type-1 DM and only 10% mortality. They stated that the this dose of Alloxan agents required for inducing DM can be toxic if it administrate by the route of intravenous (IV) [
512].
Jain and Arya in 2011 state that doses of drugs, routes of administration, are more important issue in duration and severity of diabetes and methodology in Alloxan-induced diabetic models. In this study they reported that lower doses of Alloxan at ranges of 90-140 mg/kg, ip can led to auto-reversion to normal condition [
15]. In our study, we found that Alloxan at dose of 140 mg/kg, ip and 120 mg/kg, sc is optimal dose in inducing high rate of diabetes.
As well as Chougale et al. tried to investigate on the optimization of Alloxan dose to induce stable diabetes for prolonged period. They revealed that usage of Alloxan varies from 80 mg to even ip, 200 mg/kg of body weight. They found that Alloxan given at a dose of 160 mg/kg of body weight is suitable to induce stable diabetes with minimal Alloxan toxicity [
1214].
In current study, we found that sc delivery of Alloxan drug is preferable an optimal rout in inducing high rate of diabetes in rats rather to intraperitoneal injection. This finding are in parallel to the Kalinichev, et al. findings: Kalinichev, et al. in 2008 outlined to evaluate the effects of the drug on locomotor activity and immediate early gene (IEG) induction in the rat using two routes of drug administration, ip and SC. They showed that Administration of PCP resulted in locomotor hyperactivity which was more robust and longer-lasting in animals dosed SC compared to ip-treated-animals. Differences in hyperlocomotion were paralleled by higher concentrations of phencyclidine (PCP) in the blood and in the brain of SC-treated animals compared to ip-treated animals. The differences in the concentration of PCP between the two routes of administration were detected 30 min after dosing and persisted for up to 4 h. Administration of PCP via the SC route resulted in induction of more IEGs and consistently larger magnitudes of induction than that via the ip route. They concluded that the subcutaneous administration is preferable route in rat. However other results show that ip is preferable rout in small rodents [
16].
Inoue et al compared sc injection and ip injection of D-Lucifer in for
in vivo bioluminescence imaging (BLI) to determine the utility of sc injection. They observed in sc tumors, the peak time was slightly shorter and the peak signal was greater using sc injection than using ip injection. The repeatability of determining peak signals was comparable between the two injection routes, and a good correlation was observed between them. In mice bearing both sc and ip tumors, signals from ip tumors relative to those from sc tumors were much greater using ip injection than using IV or sc injection. In the hematological malignancy model, signals from the spleen relative to those from the bone marrow were greater using ip injection than using sc injection. Inoue et al. Concluded that that in addition to rare injection failure, the ip injection of D: -luciferin led to the overestimation of signals from ip tissues. For BLI, sc injection was shown to be a convenient alternative to ip injection [
17]. We demonstrated that the STZ at dose of 40 was the preferable dose for high induction of DM with low mortality rates. In this regard Ar'Rajab and Ahren studied the long-term effects of STZ (30-70 mg/kg) on plasma glucose and insulin levels, islet morphology, and glucose-stimulated insulin secretion in rats. In addition, the protective effect of short-term (7 days) insulin treatment on STZ-induced DM was examined. STZ administration at dose levels exceeding 40 mg/kg resulted in a long term, stable hyperglycemia with no insulin response to glucose at 3 months and with a marked derangement of islet morphology (few insulin cells, accumulation of glucagon cells). In contrast, at 30 and 40 mg/kg, STZ induced a transient DM. Thus, the blood glucose levels, being elevated at days 1-7, returned to normal levels within 10 days after STZ administration and the glucose-induced insulin secretion, being absent at day I, was normal at 3 months. Furthermore, the islet morphology was also normal in these groups at 3 months. Short-term (7 days) insulin treatment normalized the long-term DM in rats given 50 mg/kg STZ, but not in rats given 60 or 70 mg/kg STZ. Thus, after insulin treatment, all rats receiving 50 mg/kg STZ returned to normoglycemia within the following 2 weeks, and the glucose-induced insulin secretion was normal after 3 months, as was islet morphology. Ar'Rajab and Ahren concluded that (a) spontaneous recovery after STZ is dose dependent and occurs in rats given 30 or 40 mg/kg of the drug, whereas at 50, 60, or 70 mg/kg, STZ induces a stable DM; and (b) a short-term (7 days) insulin treatment turns the DM induced by STZ at 50 mg/kg into a transient type, whereas the STZ-induced DM at 60 or 70 mg/kg is stable despite insulin treatment [
13], and also Srinivasan, et al. showed that the rats administered STZ at doses of 35-65 mg/kg (iv or ip) develop moderate and stable nonfasting hyperglycemia [
12].
Go to :

Conclusion
The novelty of this scientific work was that male Wistar rats which treated by a single sc injection of Alloxan at dose of 120 mg/kg and also rats that treated by ip injection of STZ at dose of 40 mg/kg developed a persistent and optimal type I DM state characterized by high rate of DM induction and low level of mortality. Further studies using different animal strain and sex and age are suggested.
Go to :

Acknowledgment
We wish to extend our sincere thanks to the late Mrs. Jamileh Rezaei. This article is financially supported "by research department of the school of medicine" and Vice Chancellors of Research at the Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grants no 1393-1-91-13500).
Go to :

Notes
Go to :

References
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2014; 37:S81. PMID:
24357215.
2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87(1):4–14. PMID:
19896746.

3. Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, Lewis SE. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007; 22(7):1871–1877. PMID:
17478459.

4. Etuk EU. Animal models for studying diabetes mellitus. Agric Biol JN Am. 2010; 1(2):130–134.
5. Federiuk IF, Casey HM, Quinn MJ, Wood MD, Ward WK. Induction of type-1 diabetes mellitus in laboratory rats by use of alloxan: route of administration, pitfalls, and insulin treatment. Comp Med. 2004; 54(3):252–257. PMID:
15253270.
6. Awai M, Narasaki M, Yamanoi Y, Seno S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am J Pathol. 1979; 95(3):663–673. PMID:
377994.
7. Chatzigeorgiou A, Halapas A, Kalafatakis K, Kamper E. The use of animal models in the study of diabetes mellitus.
In Vivo. 2009; 23(2):245–258. PMID:
19414410.
8. Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005; 22(4):359–370. PMID:
15787657.

9. King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012; 166(3):877–894. PMID:
22352879.

10. Hoftiezer V, Carpenter AM. Comparison of streptozotocin and alloxan-induced diabetes in the rat, including volumetric quantitation of the pancreatic islets. Diabetologia. 1973; 9(3):178–184. PMID:
4268550.

11. Gajdosik A, Gajdosikova A, Stefek M, Navarova J, Hozova R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999; 18:54–62. PMID:
10703720.
12. Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007; 125(3):451–472. PMID:
17496368.
13. Ar'Rajab A, Ahren B. Long-term diabetogenic effect of streptozotocin in rats. Pancreas. 1993; 8(1):50–57. PMID:
8419909.
14. Chougale AD, Panaskar SN, Gurao PM, Arvindekar AU. Optimization of Alloxan Dose is Essential to Induce Stable Diabetes for Prolonged Period. Asian J Biochem. 2007; 2(6):402–408.
15. Jain DK, Arya RK. Anomalies in alloxan-induced diabetic model: It is better to standardize it first. Indian J Pharmacol. 2011; 43(1):91. PMID:
21455436.

16. Kalinichev M, Robbins MJ, Hartfield EM, Maycox PR, Moore SH, Savage KM, Austin NE, Jones DN. Comparison between intraperitoneal and subcutaneous phencyclidine administration in Sprague-Dawley rats: a locomotor activity and gene induction study. Prog Neuropsychopharmacol Biol Psychiatry. 2008; 32(2):414–422. PMID:
17945407.

17. Inoue Y, Kiryu S, Izawa K, Watanabe M, Tojo A, Ohtomo K. Comparison of subcutaneous and intraperitoneal injection of Dluciferin for
in vivo bioluminescence imaging. Eur J Nucl Med Mol Imaging. 2009; 36(5):771–779. PMID:
19096841.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download